| |||
| Names | |||
|---|---|---|---|
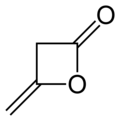
| Preferred IUPAC name 4-Methylideneoxetan-2-one | |||
| Other names γ-Methylenepropiolactone | |||
| Identifiers | |||
3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.010.562 | ||
| EC Number |
| ||
PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 2521 | ||
CompTox Dashboard (EPA) | |||
| |||
| |||
| Properties | |||
| C4H4O2 | |||
| Molar mass | 84.074 g·mol−1 | ||
| Density | 1.09 g cm−3 | ||
| Melting point | −7 °C (19 °F; 266 K) | ||
| Boiling point | 127 °C (261 °F; 400 K) | ||
| Viscosity | 0.88 mPa.s | ||
| Hazards | |||
| GHS labelling: | |||
    | |||
| Danger | |||
| H226, H301, H302, H315, H318, H330, H331, H332, H335 | |||
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P280, P284, P301+P310, P301+P312, P302+P352, P303+P361+P353, P304+P312, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P330, P332+P313, P362, P370+P378, P403+P233, P403+P235, P405, P501 | |||
| Flash point | 33 °C (91 °F; 306 K) | ||
| 275 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
Diketene is an organic compound with the molecular formula C4H4O2, and which is sometimes written as (CH2CO)2. It is formed by dimerization of ketene, H2C=C=O. Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. [1] It is a colorless liquid.