Related Research Articles

A protein kinase is a kinase which selectively modifies other proteins by covalently adding phosphates to them (phosphorylation) as opposed to kinases which modify lipids, carbohydrates, or other molecules. Phosphorylation usually results in a functional change of the target protein (substrate) by changing enzyme activity, cellular location, or association with other proteins. The human genome contains about 500 protein kinase genes and they constitute about 2% of all human genes. There are two main types of protein kinase. The great majority are serine/threonine kinases, which phosphorylate the hydroxyl groups of serines and threonines in their targets. Most of the others are tyrosine kinases, although additional types exist. Protein kinases are also found in bacteria and plants. Up to 30% of all human proteins may be modified by kinase activity, and kinases are known to regulate the majority of cellular pathways, especially those involved in signal transduction.
Serine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.

Threonine is an amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, a carboxyl group, and a side chain containing a hydroxyl group, making it a polar, uncharged amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Threonine is synthesized from aspartate in bacteria such as E. coli. It is encoded by all the codons starting AC.
In molecular biology, biosynthesis is a multi-step, enzyme-catalyzed process where substrates are converted into more complex products in living organisms. In biosynthesis, simple compounds are modified, converted into other compounds, or joined to form macromolecules. This process often consists of metabolic pathways. Some of these biosynthetic pathways are located within a single cellular organelle, while others involve enzymes that are located within multiple cellular organelles. Examples of these biosynthetic pathways include the production of lipid membrane components and nucleotides. Biosynthesis is usually synonymous with anabolism.

A serine/threonine protein kinase is a kinase enzyme, in particular a protein kinase, that phosphorylates the OH group of the amino-acid residues serine or threonine, which have similar side chains. At least 350 of the 500+ human protein kinases are serine/threonine kinases (STK).

Amino acid synthesis is the set of biochemical processes by which the amino acids are produced. The substrates for these processes are various compounds in the organism's diet or growth media. Not all organisms are able to synthesize all amino acids. For example, humans can synthesize 11 of the 20 standard amino acids. These 11 are called the non-essential amino acids).

Serine hydroxymethyltransferase (SHMT) is a pyridoxal phosphate (PLP) (Vitamin B6) dependent enzyme (EC 2.1.2.1) which plays an important role in cellular one-carbon pathways by catalyzing the reversible, simultaneous conversions of L-serine to glycine and tetrahydrofolate (THF) to 5,10-Methylenetetrahydrofolate (5,10-CH2-THF). This reaction provides the largest part of the one-carbon units available to the cell.
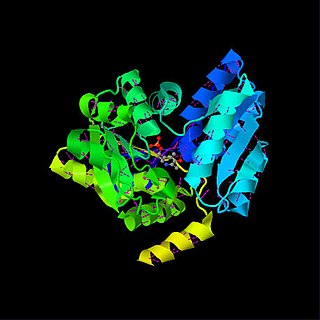
Serine dehydratase or L-serine ammonia lyase (SDH) is in the β-family of pyridoxal phosphate-dependent (PLP) enzymes. SDH is found widely in nature, but its structural and properties vary among species. SDH is found in yeast, bacteria, and the cytoplasm of mammalian hepatocytes. SDH catalyzes is the deamination of L-serine to yield pyruvate, with the release of ammonia.
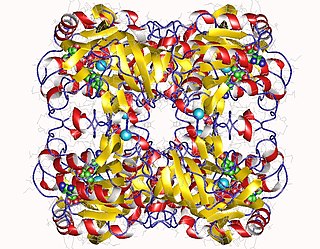
In enzymology, a L-threonine 3-dehydrogenase (EC 1.1.1.103) is an enzyme that catalyzes the chemical reaction
In enzymology, a (R)-aminopropanol dehydrogenase (EC 1.1.1.75) is an enzyme that catalyzes the chemical reaction
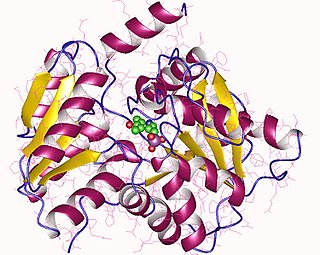
The enzyme L-serine ammonia-lyase (EC 4.3.1.17) catalyzes the chemical reaction

Threonine ammonia-lyase (EC 4.3.1.19, systematic name L-threonine ammonia-lyase (2-oxobutanoate-forming), also commonly referred to as threonine deaminase or threonine dehydratase, is an enzyme responsible for catalyzing the conversion of L-threonine into α-ketobutyrate and ammonia:
The enzyme phosphatidylserine decarboxylase (EC 4.1.1.65) catalyzes the chemical reaction

The enzyme threonine aldolase is an enzyme that catalyzes the chemical reaction
In enzymology, a serine—tRNA ligase is an enzyme that catalyzes the chemical reaction
In enzymology, a CDP-diacylglycerol—serine O-phosphatidyltransferase is an enzyme that catalyzes the chemical reaction

Glycine betaine aldehyde, often simply called betaine aldehyde, is an intermediate in the metabolism of glycine, serine and threonine. The human aldehyde dehydrogenase stimulates the transformation of betaine aldehyde to glycine betaine. Betaine aldehyde is a substrate for choline dehydrogenase (mitochondrial).
Carnitine biosynthesis is a method for the endogenous production of L-carnitine, a molecule that is essential for energy metabolism. In humans and many other animals, L-carnitine is obtained from both diet and by biosynthesis. The carnitine biosynthesis pathway is highly conserved among many eukaryotes and some prokaryotes.
D-threonine aldolase is an enzyme with systematic name D-threonine acetaldehyde-lyase (glycine-forming). This enzyme catalyses the following chemical reaction
Low-specificity L-threonine aldolase is an enzyme with systematic name L-threonine/L-allo-threonine acetaldehyde-lyase (glycine-forming). This enzyme catalyses the following chemical reaction
References
- 1 2 Liu JQ, Dairi T, Itoh N, Kataoka M, Shimizu S, Yamada H (July 1998). "Gene cloning, biochemical characterization and physiological role of a thermostable low-specificity L-threonine aldolase from Escherichia coli". European Journal of Biochemistry. 255 (1): 220–6. doi: 10.1046/j.1432-1327.1998.2550220.x . PMID 9692922.
- ↑ NCBI Unigene record Zm.80943, listing various species where enzyme homologs are present.