
In chemistry, an oxidizing agent is a substance that has the ability to oxidize other substances — in other words to cause them to lose electrons. Common oxidizing agents are oxygen, hydrogen peroxide and the halogens.

Chromate salts contain the chromate anion, CrO2−
4. Dichromate salts contain the dichromate anion, Cr
2O2−
7. They are oxoanions of chromium in the 6+ oxidation state and are moderately strong oxidizing agents. In an aqueous solution, chromate and dichromate ions can be interconvertible.

Chromite is a mineral that is an iron chromium oxide. It has a chemical formula of FeCr2O4. It is an oxide mineral belonging to the spinel group. The element magnesium can substitute for iron in variable amounts as it forms a solid solution with magnesiochromite (MgCr2O4). A substitution of the element aluminium can also occur, leading to hercynite (FeAl2O4). Chromite today is mined particularly to make stainless steel through the production of ferrochrome (FeCr), which is an iron-chromium alloy.
In inorganic chemistry, a ternary compound is a compound containing three different elements. An example is sodium phosphate, Na3PO4. The sodium ion has a charge of 1+ and the phosphate ion has a charge of 3-. Therefore, three sodium ions are needed to balance the charge of one phosphate ion. Another example of a ternary compound is calcium carbonate (CaCO3). In naming and writing the formulae for ternary compounds, rules are similar to binary compounds.
Plating is a surface covering in which a metal is deposited on a conductive surface. Plating has been done for hundreds of years; it is also critical for modern technology. Plating is used to decorate objects, for corrosion inhibition, to improve solderability, to harden, to improve wearability, to reduce friction, to improve paint adhesion, to alter conductivity, to improve IR reflectivity, for radiation shielding, and for other purposes. Jewelry typically uses plating to give a silver or gold finish.

Chromium trioxide is an inorganic compound with the formula CrO3. It is the acidic anhydride of chromic acid, and is sometimes marketed under the same name. This compound is a dark-purple solid under anhydrous conditions, bright orange when wet and which dissolves in water concomitant with hydrolysis. Millions of kilograms are produced annually, mainly for electroplating. Chromium trioxide is a powerful oxidiser and a suspected carcinogen.
Metal fume fever, also known as brass founders' ague, brass shakes, zinc shakes, galvie flu, metal dust fever, Welding Shivers, or Monday morning fever, is an illness primarily caused by exposure to chemicals such as zinc oxide (ZnO), aluminium oxide (Al2O3), or magnesium oxide (MgO) which are produced as byproducts in the fumes that result when certain metals are heated. Other common sources are fuming silver, gold, platinum, chromium (from stainless steel), nickel, arsenic, manganese, beryllium, cadmium, cobalt, lead, selenium, and zinc.
Copper chromite is an inorganic compound with the formula Cu2Cr2O5 which is used to catalyze reactions in organic synthesis.

Chromium carbide is a ceramic compound that exists in several different chemical compositions: Cr3C2, Cr7C3,and Cr23C6. At standard conditions it exists as a gray solid. It is extremely hard and corrosion resistant. It is also a refractory compound, which means that it retains its strength at high temperatures as well. These properties make it useful as an additive to metal alloys. When chromium carbide crystals are integrated into the surface of a metal it improves the wear resistance and corrosion resistance of the metal, and maintains these properties at elevated temperatures. The hardest and most commonly used composition for this purpose is Cr3C2.

Nickel(II) oxide is the chemical compound with the formula NiO. It is notable as being the only well-characterized oxide of nickel. The mineralogical form of NiO, bunsenite, is very rare. It is classified as a basic metal oxide. Several million kilograms are produced in varying quality annually, mainly as an intermediate in the production of nickel alloys.

Ammonium dichromate is the inorganic compound with the formula (NH4)2Cr2O7. In this compound, as in all chromates and dichromates, chromium is in a +6 oxidation state, commonly known as hexavalent chromium. It is a salt consisting of ammonium ions and dichromate ions.

Sodium chromate is the inorganic compound with the formula Na2CrO4. It exists as a yellow hygroscopic solid, which can form tetra-, hexa-, and decahydrates. It is an intermediate in the extraction of chromium from its ores. Sodium chromate, like other hexavalent chromium compounds, is toxic and carcinogenic.

Chromium(VI) peroxide (CrO5) or chromium oxide peroxide is an unstable compound formed by the addition of acidified hydrogen peroxide solutions to solutions of metal chromates or dichromates, such as sodium chromate or potassium dichromate. The generally yellow chromates or orange dichromates turn to dark blue as chromium(VI) peroxide is formed. Chromate or dichromate reacts with hydrogen peroxide and an acid to give chromium peroxide and water.

In chemistry the term chromite has been used in two contexts. Under IUPAC naming conventions, chromate(III) is preferred to chromite.
- For compounds containing an oxyanion of chromium in oxidation state 3
- For other compounds of chromium(III) as a means of distinguishing a chemical species such as hexacyanochromite(III). [Cr(CN)6]3− from an analogous compound in which chromium is a different oxidation state.

The Jones oxidation is an organic reaction for the oxidation of primary and secondary alcohols to carboxylic acids and ketones, respectively. It is named after its discoverer, Sir Ewart Jones.
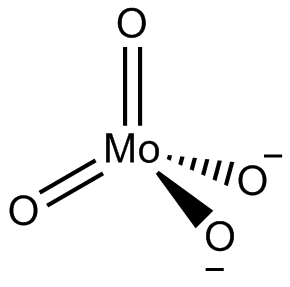
In chemistry a molybdate is a compound containing an oxoanion with molybdenum in its highest oxidation state of 6. Molybdenum can form a very large range of such oxoanions which can be discrete structures or polymeric extended structures, although the latter are only found in the solid state.The larger oxoanions are members of group of compounds termed polyoxometalates, and because they contain only one type of metal atom are often called isopolymetalates. The discrete molybdenum oxoanions range in size from the simplest MoO2−
4, found in potassium molybdate up to extremely large structures found in isopoly-molybdenum blues that contain for example 154 Mo atoms. The behaviour of molybdenum is different from the other elements in group 6. Chromium only forms the chromates, CrO2−
4, Cr
2O2−
7, Cr
3O2−
10 and Cr
4O2−
13 ions which are all based on tetrahedral chromium. Tungsten is similar to molybdenum and forms many tungstates containing 6 coordinate tungsten.
A chromate ester is a chemical structure that contains a chromium atom (symbol Cr) in a +6 oxidation state that is connected via an oxygen (O) linkage to a carbon (C) atom. The Cr itself is in its chromate form, with several oxygens attached, and the Cr–O–C attachment makes this chemical group structurally similar to other ester functional groups. They can be synthesized from various chromium(VI) metal compounds, such as CrO3, chromium chloride complexes, and aqueous chromate ions, and tend to react via redox reactions to liberate chromium(IV).
The spinels are any of a class of minerals of general formulation AB
2X
4 which crystallise in the cubic (isometric) crystal system, with the X anions arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice. Although the charges of A and B in the prototypical spinel structure are +2 and +3, respectively, other combinations incorporating divalent, trivalent, or tetravalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon, are also possible. The anion is normally oxygen; when other chalcogenides constitute the anion sublattice the structure is referred to as a thiospinel.













