
Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production. Coenzyme A consists of a β-mercaptoethylamine group linked to the vitamin pantothenic acid (B5) through an amide linkage and 3'-phosphorylated ADP. The acetyl group of acetyl-CoA is linked to the sulfhydryl substituent of the β-mercaptoethylamine group. This thioester linkage is a "high energy" bond, which is particularly reactive. Hydrolysis of the thioester bond is exergonic (−31.5 kJ/mol).
Gluconeogenesis (GNG) is a metabolic pathway that results in the generation of glucose from certain non-carbohydrate carbon substrates. It is a ubiquitous process, present in plants, animals, fungi, bacteria, and other microorganisms. In vertebrates, gluconeogenesis occurs mainly in the liver and, to a lesser extent, in the cortex of the kidneys. It is one of two primary mechanisms – the other being degradation of glycogen (glycogenolysis) – used by humans and many other animals to maintain blood sugar levels, avoiding low levels (hypoglycemia). In ruminants, because dietary carbohydrates tend to be metabolized by rumen organisms, gluconeogenesis occurs regardless of fasting, low-carbohydrate diets, exercise, etc. In many other animals, the process occurs during periods of fasting, starvation, low-carbohydrate diets, or intense exercise.

Biological carbon fixation or сarbon assimilation is the process by which inorganic carbon is converted to organic compounds by living organisms. The compounds are then used to store energy and as structure for other biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use a process called chemosynthesis in the absence of sunlight.

Oxaloacetic acid (also known as oxalacetic acid or OAA) is a crystalline organic compound with the chemical formula HO2CC(O)CH2CO2H. Oxaloacetic acid, in the form of its conjugate base oxaloacetate, is a metabolic intermediate in many processes that occur in animals. It takes part in gluconeogenesis, the urea cycle, the glyoxylate cycle, amino acid synthesis, fatty acid synthesis and the citric acid cycle.
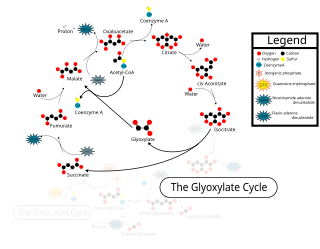
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.
The enzyme L-erythro-3-hydroxyaspartate aldolase catalyzes the chemical reaction
The enzyme 4-hydroxy-2-oxoglutarate aldolase catalyzes the chemical reaction
The enzyme citrate (pro-3S)-lyase catalyzes the chemical reaction
The enzyme citryl-CoA lyase catalyzes the chemical reaction

Isocitrate lyase, or ICL, is an enzyme in the glyoxylate cycle that catalyzes the cleavage of isocitrate to succinate and glyoxylate. Together with malate synthase, it bypasses the two decarboxylation steps of the tricarboxylic acid cycle and is used by bacteria, fungi, and plants.
The enzyme malyl-CoA lyase catalyzes the chemical reaction

The enzyme methylisocitrate lyase catalyzes the chemical reaction
In enzymology, an oxalate decarboxylase (EC 4.1.1.2) is an oxalate degrading enzyme that catalyzes the chemical reaction
The enzyme L(+)-tartrate dehydratase (EC 4.2.1.32) catalyzes the chemical reaction
In enzymology, a 2-hydroxyglutarate synthase (EC 2.3.3.11) is an enzyme that catalyzes the chemical reaction

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
Glyoxylate and dicarboxylate metabolism describes a variety of reactions involving glyoxylate or dicarboxylates. Glyoxylate is the conjugate base of glyoxylic acid, and within a buffered environment of known pH such as the cell cytoplasm these terms can be used almost interchangeably, as the gain or loss of a hydrogen ion is all that distinguishes them, and this can occur in the aqueous environment at any time. Likewise dicarboxylates are the conjugate bases of dicarboxylic acids, a general class of organic compounds containing two carboxylic acid groups, such as oxalic acid or succinic acid.
Isocitrate lyase family is a family of evolutionarily related proteins.

In molecular biology, the citrate synthase family of proteins includes the enzymes citrate synthase EC 2.3.3.1, and the related enzymes 2-methylcitrate synthase EC 2.3.3.5 and ATP citrate lyase EC 2.3.3.8.
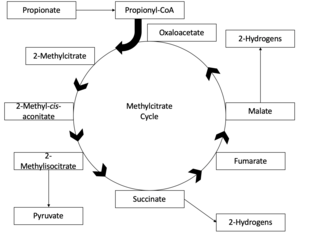
The methylcitrate cycle, or the MCC, is the mechanism by which propionyl-CoA is formed, generated by β-oxidation of odd-chain fatty acids, and broken down to its final products, succinate and pyruvate. The methylcitrate cycle is closely related to both the citric acid cycle and the glyoxylate cycle, in that they share substrates, enzymes and products. The methylcitrate cycle functions overall to detoxify bacteria of toxic propionyl-CoA, and plays an essential role in propionate metabolism in bacteria. Incomplete propionyl-CoA metabolism may lead to the buildup of toxic metabolites in bacteria, and thus the function of the methylcitrate cycle is an important biological process.











