Related Research Articles

The thalamus is a large mass of gray matter located in the dorsal part of the diencephalon. Nerve fibers project out of the thalamus to the cerebral cortex in all directions, allowing hub-like exchanges of information. It has several functions, such as the relaying of sensory signals, including motor signals to the cerebral cortex and the regulation of consciousness, sleep, and alertness.
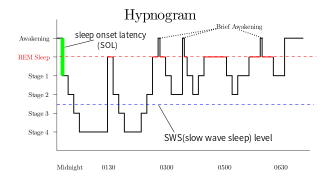
Rapid eye movement sleep is a unique phase of sleep in mammals and birds, characterized by random rapid movement of the eyes, accompanied by low muscle tone throughout the body, and the propensity of the sleeper to dream vividly.

The brainstem is the stalk-like part of the brain that interconnects the cerebrum and diencephalon with the spinal cord. In the human brain, the brainstem is composed of the midbrain, the pons, and the medulla oblongata. The midbrain is continuous with the thalamus of the diencephalon through the tentorial notch.
The internal capsule is a white matter structure situated in the inferomedial part of each cerebral hemisphere of the brain. It carries information past the basal ganglia, separating the caudate nucleus and the thalamus from the putamen and the globus pallidus. The internal capsule contains both ascending and descending axons, going to and coming from the cerebral cortex. It also separates the caudate nucleus and the putamen in the dorsal striatum, a brain region involved in motor and reward pathways.

The auditory system is the sensory system for the sense of hearing. It includes both the sensory organs and the auditory parts of the sensory system.

The subthalamic nucleus (STN) is a small lens-shaped nucleus in the brain where it is, from a functional point of view, part of the basal ganglia system. In terms of anatomy, it is the major part of the subthalamus. As suggested by its name, the subthalamic nucleus is located ventral to the thalamus. It is also dorsal to the substantia nigra and medial to the internal capsule. It was first described by Jules Bernard Luys in 1865, and the term corpus Luysi or Luys' body is still sometimes used.

In neuroanatomy, the pretectal area, or pretectum, is a midbrain structure composed of seven nuclei and comprises part of the subcortical visual system. Through reciprocal bilateral projections from the retina, it is involved primarily in mediating behavioral responses to acute changes in ambient light such as the pupillary light reflex, the optokinetic reflex, and temporary changes to the circadian rhythm. In addition to the pretectum's role in the visual system, the anterior pretectal nucleus has been found to mediate somatosensory and nociceptive information.

The reticular formation is a set of interconnected nuclei that are located throughout the brainstem. It is not anatomically well defined, because it includes neurons located in different parts of the brain. The neurons of the reticular formation make up a complex set of networks in the core of the brainstem that extend from the upper part of the midbrain to the lower part of the medulla oblongata. The reticular formation includes ascending pathways to the cortex in the ascending reticular activating system (ARAS) and descending pathways to the spinal cord via the reticulospinal tracts.

The pontine tegmentum, or dorsal pons, is located within the brainstem, and is one of two parts of the pons, the other being the ventral pons or basilar part of the pons. The pontine tegmentum can be defined in contrast to the basilar pons: basilar pons contains the corticospinal tract running craniocaudally and can be considered the rostral extension of the ventral medulla oblongata; however, basilar pons is distinguished from ventral medulla oblongata in that it contains additional transverse pontine fibres that continue laterally to become the middle cerebellar peduncle. The pontine tegmentum is all the material dorsal from the basilar pons to the fourth ventricle. Along with the dorsal surface of the medulla, it forms part of the rhomboid fossa – the floor of the fourth ventricle.
The pedunculopontine nucleus (PPN) or pedunculopontine tegmental nucleus is a collection of neurons located in the upper pons in the brainstem. It lies caudal to the substantia nigra and adjacent to the superior cerebellar peduncle. It has two divisions of subnuclei; the pars compacta containing mainly cholinergic neurons, and the pars dissipata containing mainly glutamatergic neurons and some non-cholinergic neurons. The pedunculopontine nucleus is one of the main components of the reticular activating system. It was first described in 1909 by Louis Jacobsohn-Lask, a German neuroanatomist.

The medial geniculate nucleus (MGN) or medial geniculate body (MGB) is part of the auditory thalamus and represents the thalamic relay between the inferior colliculus (IC) and the auditory cortex (AC). It is made up of a number of sub-nuclei that are distinguished by their neuronal morphology and density, by their afferent and efferent connections, and by the coding properties of their neurons. It is thought that the MGN influences the direction and maintenance of attention.

Slow-wave sleep (SWS), often referred to as deep sleep, consists of stage three of non-rapid eye movement sleep. It usually lasts between 70 and 90 minutes and takes place during the first hours of the night. Initially, SWS consisted of both Stage 3, which has 20–50 percent delta wave activity, and Stage 4, which has more than 50 percent delta wave activity.
The zona incerta (ZI) is a horizontally elongated region of gray matter in the subthalamus below the thalamus. Its connections project extensively over the brain from the cerebral cortex down into the spinal cord.

The cochlear nuclear (CN) complex comprises two cranial nerve nuclei in the human brainstem, the ventral cochlear nucleus (VCN) and the dorsal cochlear nucleus (DCN). The ventral cochlear nucleus is unlayered whereas the dorsal cochlear nucleus is layered. Auditory nerve fibers, fibers that travel through the auditory nerve carry information from the inner ear, the cochlea, on the same side of the head, to the nerve root in the ventral cochlear nucleus. At the nerve root the fibers branch to innervate the ventral cochlear nucleus and the deep layer of the dorsal cochlear nucleus. All acoustic information thus enters the brain through the cochlear nuclei, where the processing of acoustic information begins. The outputs from the cochlear nuclei are received in higher regions of the auditory brainstem.
Recurrent thalamo-cortical resonance is an observed phenomenon of oscillatory neural activity between the thalamus and various cortical regions of the brain. It is proposed by Rodolfo Llinas and others as a theory for the integration of sensory information into the whole of perception in the brain. Thalamocortical oscillation is proposed to be a mechanism of synchronization between different cortical regions of the brain, a process known as temporal binding. This is possible through the existence of thalamocortical networks, groupings of thalamic and cortical cells that exhibit oscillatory properties.
Low-threshold spikes (LTS) refer to membrane depolarizations by the T-type calcium channel. LTS occur at low, negative, membrane depolarizations. They often follow a membrane hyperpolarization, which can be the result of decreased excitability or increased inhibition. LTS result in the neuron reaching the threshold for an action potential. LTS is a large depolarization due to an increase in Ca2+ conductance, so LTS is mediated by calcium (Ca2+) conductance. The spike is typically crowned by a burst of two to seven action potentials, which is known as a low-threshold burst. LTS are voltage dependent and are inactivated if the cell's resting membrane potential is more depolarized than −60mV. LTS are deinactivated, or recover from inactivation, if the cell is hyperpolarized and can be activated by depolarizing inputs, such as excitatory postsynaptic potentials (EPSP). LTS were discovered by Rodolfo Llinás and coworkers in the 1980s.

The anatomy of the cerebellum can be viewed at three levels. At the level of gross anatomy, the cerebellum consists of a tightly folded and crumpled layer of cortex, with white matter underneath, several deep nuclei embedded in the white matter, and a fluid-filled ventricle in the middle. At the intermediate level, the cerebellum and its auxiliary structures can be broken down into several hundred or thousand independently functioning modules or compartments known as microzones. At the microscopic level, each module consists of the same small set of neuronal elements, laid out with a highly stereotyped geometry.

The relationship between sleep and memory has been studied since at least the early 19th century. Memory, the cognitive process of storing and retrieving past experiences, learning and recognition, is a product of brain plasticity, the structural changes within synapses that create associations between stimuli. Stimuli are encoded within milliseconds; however, the long-term maintenance of memories can take additional minutes, days, or even years to fully consolidate and become a stable memory that is accessible. Therefore, the formation of a specific memory occurs rapidly, but the evolution of a memory is often an ongoing process.
The activation-synthesis hypothesis, proposed by Harvard University psychiatrists John Allan Hobson and Robert McCarley, is a neurobiological theory of dreams first published in the American Journal of Psychiatry in December 1977. The differences in neuronal activity of the brainstem during waking and REM sleep were observed, and the hypothesis proposes that dreams result from brain activation during REM sleep. Since then, the hypothesis has undergone an evolution as technology and experimental equipment has become more precise. Currently, a three-dimensional model called AIM Model, described below, is used to determine the different states of the brain over the course of the day and night. The AIM Model introduces a new hypothesis that primary consciousness is an important building block on which secondary consciousness is constructed.
The parafacial zone (PZ) is a brain structure located in the brainstem within the medulla oblongata believed to be heavily responsible for non-rapid eye movement (non-REM) sleep regulation, specifically for inducing slow-wave sleep.
References
- 1 2 Gott, Jarrod A.; Liley, David T. J.; Hobson, J. Allan (2017). "Towards a Functional Understanding of PGO Waves". Frontiers in Human Neuroscience. 11: 89. doi: 10.3389/fnhum.2017.00089 . ISSN 1662-5161. PMC 5334507 . PMID 28316568.
- ↑ Lim, Andrew S.; Lozano, Andres M.; Moro, Elena; Hamani, Clement; Hutchison, William D.; Dostrovsky, Jonathan O.; Lang, Anthony E.; Wennberg, Richard A.; Murray, Brian J. (2007-07-01). "Characterization of REM-Sleep Associated Ponto-Geniculo-Occipital Waves in the Human Pons". Sleep. 30 (7): 823–827. doi:10.1093/sleep/30.7.823. ISSN 0161-8105. PMC 1978372 . PMID 17682651.
- ↑ Jouvet, M., Michel, F., and Courjon, J. 1959. L'activite electrique du rhinencephale au cours dusommeil chez le chat. C.R. Soc. Biol. 153:101–105.
- ↑ Brooks, D.C., and Bizzi, E. 1963. Brain stem electrical activity during deep sleep. Arch. Ital. Biol.101:648–665.
- ↑ Stern, W.C., Forbes, W.B., and Morgane, P.J. 1974. Absence of ponto-geniculo-occipital (PGO) spikes in rats. Physiol. Behav. 12:293–295.
- 1 2 3 4 5 Datta S. 1997. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cellular and Molecular Neurobiology 17:341–65
- ↑ Fernández-Mendoza J., Lozano B., Seijo F., Fernández-González F., Vela-Bueno A. 2006. Subthalamic nucleus activity during human REM sleep: the PGO-like waves. J Sleep Res 2006;15:243.
- ↑ Lim, A.S.; Lozano, A.M.; Moro, E.; Hamani, C.; Hutchison, W.D.; et al. (2007b). "Characterization of REM-sleep associated ponto-geniculo-occipital waves in the human pons". Sleep. 30 (7): 823–7. doi:10.1093/sleep/30.7.823. PMC 1978372 . PMID 17682651.
- 1 2 Datta, S.; Hobson, J.A. (1994). "Neuronal activity in the caudo-lateral peribrachial pons:Relationship to PGO waves and rapid eye movements". J. Neurophysiol. 71 (1): 95–109. doi:10.1152/jn.1994.71.1.95. PMID 8158244.
- ↑ Williams, J.A.; Reiner, P.B. (1993). "Noradrenaline hyperpolarizes identified rat mesopontine cholinergic neurons in vitro". J. Neurosci. 13 (9): 3878–3883. doi: 10.1523/jneurosci.13-09-03878.1993 . PMC 6576458 . PMID 8103553.
- ↑ Brooks, D. C.; Gershon, M. D. (1977-01-01). "Amine repletion in the reserpinized cat: effect upon PGO waves and REM sleep". Electroencephalography and Clinical Neurophysiology. 42 (1): 35–47. doi:10.1016/0013-4694(77)90149-3. ISSN 0013-4694. PMID 64348.
- ↑ Steriade, M., Datta, S., Pare, D., Oakson, G., and Currodossi, R. (1990a). Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamortical systems. J. Heurosci. 10:2541–2559.
- ↑ Leonard, T.O.; Lydic, R. (1995). "Nitric oxide synthase inhibition decreases pontine acetylcholinerelease". NeuroReport. 6 (11): 1525–1529. doi:10.1097/00001756-199507310-00015. PMID 7579140. S2CID 10368008.
- ↑ Morrison, A.R., and Pompeiano, O. (1966). Vestibular influences during sleep. IV. Functional relations between the vestibular nsleep. Arch. Ital. Biol. 104:425–458.
- ↑ Calvo, J.M.; Badillo, S.; Morales-Ramirez, M.; Palacios-Salas, P. (1987). "The role of temporal lobe amygdala in ponto-geniculo-occipital activity and sleep organization in cats". Brain Res. 403 (1): 22–30. doi:10.1016/0006-8993(87)90118-1. PMID 3828815. S2CID 27712179.
- ↑ Siegel, J.M. (2005). "Clues to the functions of mammalian sleep". Nature. 437 (7063): 1264–71. Bibcode:2005Natur.437.1264S. doi:10.1038/nature04285. PMC 8760626 . PMID 16251951.
- ↑ Callaway C.W., Lydic R., Baghdoyan H.A., Hobson J.A. 1987. Pontogeniculoocipital Waves – Spontaneous Visual-System ACtivity During Rapid Eye-Movement Sleep. Cellular and Molecular Neurobiology 7:105–49
- ↑ Bowker, R.M., and Morrison, A.R. 1977. The PGO spikes: An indicator of hyperalertness. In Sleep Research, (W.P. Koella and P. Levin, Eds.), Karger, Basel, pp. 23–77.
- 1 2 Fernández-Mendoza J., Lozano B., Seijo F., Santamarta-Liébana E., Ramos-Platón M.J., Vela-Bueno A., Fernández-González F. 2009. Evidence of subthalamic pgo-like waves during rem sleep in humans: a deep brain polysomnographic study. SLEEP 32(9):1117–26.
- ↑ Datta, S (2006). "Activation of phasic pontine-wave generator: a mechanism for sleep-dependent memory processing". Sleep Biol. Rhythms. 4: 16–26. doi:10.1111/j.1479-8425.2006.00202.x. S2CID 144514044.
- ↑ Datta, S.; Saha, S.; Prutzman, S.L.; Mullins, O.J.; Mavanji, V. (2005). "Pontine-wave generator activation-dependent memory processing of avoidance learning involves the dorsal hippocampus in the rat". J. Neurosci. Res. 80 (5): 727–737. doi:10.1002/jnr.20501. PMC 1224707 . PMID 15880522.
- ↑ Hobson, J.A.; Pace-Schott, E.F.; Stickgold, R. (2000). "Dreaming and the brain: Toward a cognitive neuroscience of conscious states". Behavioral and Brain Sciences. 23 (6): 793–842, discussion 904–1121. doi:10.1017/S0140525X00003976. PMID 11515143. S2CID 14104546.
- ↑ Gott, J.A.; Liley, D.T.J.; Hobson, J.A. (2017). "Towards a Functional Understanding of PGO Waves". Front. Hum. Neurosci. 11: 89. doi: 10.3389/fnhum.2017.00089 . PMC 5334507 . PMID 28316568.