
A calliope is a North American musical instrument that produces sound by sending a gas, originally steam or, more recently, compressed air, through large whistles—originally locomotive whistles.

An aerophone is a musical instrument that produces sound primarily by causing a body of air to vibrate, without the use of strings or membranes, and without the vibration of the instrument itself adding considerably to the sound.
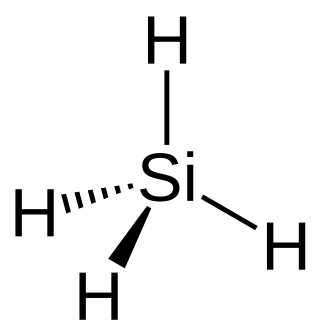
Silane (Silicane) is an inorganic compound with chemical formula SiH4. It is a colorless, pyrophoric gas with a sharp, repulsive, pungent smell, somewhat similar to that of acetic acid. Silane is of practical interest as a precursor to elemental silicon. Silanes with alkyl groups are effective water repellents for mineral surfaces such as concrete and masonry. Silanes with both organic and inorganic attachments are used as coupling agents. They are commonly used to apply coatings to surfaces or as an adhesion promoter.

Fart lighting, also known as pyroflatulence or flatus ignition, is the practice of igniting the gases produced by flatulence. The resulting flame is often of a blue hue hence the act being known colloquially as a "blue angel", "blue dart" or in Australia, a "blue flame". The fact that flatus is flammable and the actual combustion of it through this practice gives rise to much humorous derivation. Other colors of flame such as orange and yellow are possible depending on the mixture of gases formed in the colon.
A pulse detonation engine (PDE) is a type of propulsion system that uses detonation waves to combust the fuel and oxidizer mixture.

A rocket engine is a reaction engine, producing thrust in accordance with Newton's third law by ejecting reaction mass rearward, usually a high-speed jet of high-temperature gas produced by the combustion of rocket propellants stored inside the rocket. However, non-combusting forms such as cold gas thrusters and nuclear thermal rockets also exist. Rocket vehicles carry their own oxidiser, unlike most combustion engines, so rocket engines can be used in a vacuum, and they can achieve great speed, beyond escape velocity. Vehicles commonly propelled by rocket engines include missiles, artillery shells, ballistic missiles and rockets of any size, from tiny fireworks to man-sized weapons to huge spaceships.

Deflagration is subsonic combustion in which a pre-mixed flame propagates through an explosive or a mixture of fuel and oxidizer. Deflagrations in high and low explosives or fuel–oxidizer mixtures may transition to a detonation depending upon confinement and other factors. Most fires found in daily life are diffusion flames. Deflagrations with flame speeds in the range of 1 m/s differ from detonations which propagate supersonically with detonation velocities in the range of km/s.

Detonation is a type of combustion involving a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations propagate supersonically through shock waves with speeds about 1 km/sec and differ from deflagrations which have subsonic flame speeds about 1 m/sec. Detonation may form from an explosion of fuel-oxidizer mixture. Compared with deflagration, detonation doesn't need to have an external oxidizer. Oxidizers and fuel mix when deflagration occurs. Detonation is more destructive than deflagrations. In detonation, the flame front travels through the air-fuel faster than sound; while in deflagration, the flame front travels through the air-fuel slower than sound.
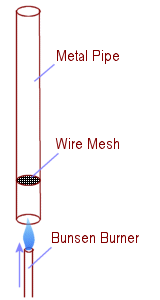
The Rijke tube is a cylindrical tube with both ends open, inside of which a heat source is placed that turns heat into sound, by creating a self-amplifying standing wave, due to thermo-acoustic instability. It is an entertaining phenomenon in acoustics and is an excellent example of resonance.

A shock tube is an instrument used to replicate and direct blast waves at a sensor or model in order to simulate explosions and their effects, usually on a smaller scale. Shock tubes can also be used to study aerodynamic flow under a wide range of temperatures and pressures that are difficult to obtain in other types of testing facilities. Shock tubes are also used to investigate compressible flow phenomena and gas phase combustion reactions. More recently, shock tubes have been used in biomedical research to study how biological specimens are affected by blast waves.
A safety lamp is any of several types of lamp that provides illumination in places such as coal mines where the air may carry coal dust or a build-up of flammable gases, which may explode if ignited, possibly by an electric spark. Until the development of effective electric lamps in the early 1900s, miners used flame lamps to provide illumination. Open flame lamps could ignite flammable gases which collected in mines, causing explosions; safety lamps were developed to enclose the flame to prevent it from igniting the explosive gases. Flame safety lamps have been replaced for lighting in mining with sealed explosion-proof electric lights, but continue to be used to detect gases.
Pressure piling is a phenomenon related to combustion of gases in a tube or long vessel. When a flame front propagates along a tube, the unburned gases ahead of the front are compressed, and hence heated. The amount of compression varies depending on the geometry and can range from twice to eight times the initial pressure. Where multiple vessels are connected by piping, ignition of gases in one vessel and pressure piling may result in a deflagration to detonation transition and very large explosion pressure.
Explosive velocity, also known as detonation velocity or velocity of detonation (VoD), is the velocity at which the shock wave front travels through a detonated explosive. Explosive velocities are always higher than the local speed of sound in the material.

Oxyhydrogen is a mixture of hydrogen (H2) and oxygen (O2) gases. This gaseous mixture is used for torches to process refractory materials and was the first gaseous mixture used for welding. Theoretically, a ratio of 2:1 hydrogen:oxygen is enough to achieve maximum efficiency; in practice a ratio 4:1 or 5:1 is needed to avoid an oxidizing flame.
Mixtures of dispersed combustible materials and oxygen in the air will burn only if the fuel concentration lies within well-defined lower and upper bounds determined experimentally, referred to as flammability limits or explosive limits. Combustion can range in violence from deflagration through detonation.

Thermal spraying techniques are coating processes in which melted materials are sprayed onto a surface. The "feedstock" is heated by electrical or chemical means.

A splint is a simple piece of equipment used in scientific laboratories. Splints are typically long, thin strips of wood, about 6 inches (15 cm) long and ¼ inch (6 mm) wide, and are consumable but inexpensive. They are typically used for tasks such as lighting bunsen burners, as the length of the splint allows a flame to be lit without risk to the user's hand, should the burner flare back. Another use for splints are chemical identification of various gases, and splints are also used to teach simple chemical principles in schools and homes.
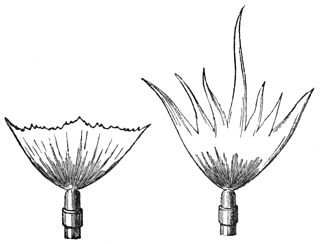
A sensitive flame is a gas flame which under suitable adjustment of pressure resonates readily with sounds or air vibrations in the vicinity. Noticed by both the American scientist John LeConte and the English physicist William Fletcher Barrett, they recorded the effect that a shrill note had upon a gas flame issuing from a tapering jet. The phenomenon caught the attention of the Irish physicist John Tyndall who gave a lecture on the process to the Royal Institution in January 1867.
Traditional French musical instruments, known as instruments traditionnels in French, are musical instruments used in the traditional folk music of France. They comprise a range of string, wind, and percussion instruments.
A Zeldovich spontaneous wave, also referred to as Zeldovich gradient mechanism, is a reaction wave that propagates spontaneously in a reacting medium with a nonuniform initial temperature distribution when there is no interaction between different fluid elements. The concept was put forward by Yakov Zeldovich in 1980, based on his earlier work with his coworkers. The spontaneous wave is different from the other two conventional combustion waves, namely the subsonic deflagrations and supersonic detonations. The wave, although strictly speaking unrealistic because gasdynamic effects are neglected, is often cited to explain the yet-unsolved problem of deflagration to detonation transition (DDT).















