
Ramalina is a genus of greenish fruticose lichens that grow in the form of flattened, strap-like branches. Members of the genus are commonly called strap lichens or cartilage lichens. Apothecia are lecanorine.

Acanthothecis is a genus of lichen-forming fungi in the family Graphidaceae. The genus was circumscribed by Frederick Edward Clements in 1909.

Parmotrema is a genus of lichen belonging to the family Parmeliaceae. It is a large genus, containing an estimated 300 species, with a centre of diversity in subtropical regions of South America and the Pacific Islands.
Psiloparmelia is a genus of lichen belonging to the family Parmeliaceae. It contains 13 Southern Hemisphere species, most of which are found growing on rocks at high elevations in South America. There are several characteristic features of the genus that are used to distinguish it from the morphologically similar genera, such as Arctoparmelia, Flavoparmelia, and Xanthoparmelia. These include a dark, velvety lower thallus surface that usually lacks rhizines, a negative test for lichenan, and a high concentration of usnic acid and atranorin in the cortex.

Niebla is a genus of yellow-green fruticose lichens that grow on rocks, trees, and shrubs within the fog zone of coastal North America, or more narrowly defined to occur on rocks and soil along the Pacific Coast from Mendocino County in California south to Baja California Sur.
André Aptroot is a Dutch mycologist and lichenologist.
Bulbothrix meizospora is a species of foliose lichen in the family Parmeliaceae. It is found in Africa, Asia, and South America, where it grows on tree bark.
Helge Thorsten Lumbsch is a German-born lichenologist living in the United States. His research interests include the phylogeny, taxonomy, and phylogeography of lichen-forming fungi; lichen diversity; lichen chemistry and chemotaxonomy. He is the Associate Curator and Head of Cryptogams and Chair of the Department of Botany at the Field Museum of Natural History.

Constipatic acid is a fatty acid found in several lichen species. It was isolated, identified, and named by Douglas Chester and John Alan Elix in a 1979 publication. The compound was extracted from the Australian leafy lichen called Xanthoparmelia constipata, which was collected on schist boulders west of Springton, South Australia. The related compounds protoconstipatic acid and dehydroconstipatic acid were also reported concurrently. Syo Kurokawa and Rex Filson had previously detected the compounds using thin-layer chromatography when they formally described the lichen as a new species in 1975, but had not characterised them chemically.
Acanthothecis salazinica is a species of script lichen in the family Graphidaceae. Found in Panama, it was described as a new species in 2013 by Pieter van den Boom and Harrie J. Sipman. The type specimen was collected near Paraíso, Panamá Province, close to the botanical garden in the Summit Park. Here it was growing on the bark of a cultivated Parmentiera cereifera tree. The lichen contains the secondary chemical salazinic acid, for which it is named. Acanthothecis subclavulifera is quite similar in morphology, but it contains protocetraric acid rather than salazinic acid and it has a different ascospore structure.
Myelochroa salazinica is a species of foliose lichen in the family Parmeliaceae. Found in China, it was described as a new species in 2001 by Sheng-Lan Wang, Jian-Bin Chen, and John Alan Elix.
Pertusaria salazinica is a species of crustose lichen in the family Pertusariaceae. Found in Australia, it was described as a new species in 2017 by lichenologists Alan Archer and John Alan Elix. The type specimen was collected in Tully Gorge National Park (Queensland) at an altitude of 885 m (2,904 ft). Here, in a montane rainforest, it was found growing on a rotting log. The specific epithet refers to the presence of salazinic acid, a major secondary compound in the lichen. It also contains norstictic acid as a major metabolite, and connorstictic acid as a minor metabolite. Pertusaria salazinica is only known from the type specimen.
Phaeographis salazinica is a species of script lichen in the family Graphidaceae. Found in the Solomon Islands, the lichen was first described as a new species in 2003 by Australian lichenologist Alan W. Archer. He named it Phaeographis salazinica, with the specific epithet referring to the presence of the compound salazinic acid as its major secondary compound. The lichen also contains trace amounts of consalazinic acid, connorstictic acid, norstictic acid, subnorstictic acid, protocetraric acid, and methyl norstictate. The type specimen was collected near Tatamba on Tanabuli Island. The main morphological characteristics of Phaeographis salazinica are the conspicuous lirellae, and the large brown muriform ascospores. Archer transferred the taxon to the genus Phaeographis in 2007.
Xanthoparmelia salazinica is a species of lichen in the family Parmeliaceae. Found in South Africa, it was described as a new species in 1989 by American lichenologist Mason Hale. He classified it in Karoowia, a genus that has since been placed in synonymy with Xanthoparmelia following molecular phylogenetic analysis published in 2010.
Carbacanthographis salazinica is a species of script lichen in the family Graphidaceae. Found in Australia, it was described as a new species in 2001 by lichenologist Alan Archer. The type specimen was collected by Archer in Conglomerate State Forest. Here the lichen was found growing on the bark of a palm tree. Its thallus is thin and grayish-green, with conspicuous white lirellae measuring 1–4 mm long. The specific epithet refers to salazinic acid, the presence of which is a distinguishing characteristic of this species. The lichen also has trace amounts of other secondary chemicals, including consalazinic acid, norstictic acid, and protocetraric acid. In 2005 Archer transferred the taxon to genus Carbacanthographis.
Parmotrema lichexanthonicum is a species of foliose lichen in the family Parmeliaceae. Found in Brazil, it was formally described as a new species in 1997 by Sionara Eliasaro and Mónica Adler. The type specimen was collected by the first author from the Serra do Cipó ; here the lichen was found growing on a rock. The specific epithet lichexanthonicum refers to the presence of the secondary compound lichexanthone in the medulla of the lichen. Other compounds in the lichen are the depsidone salazinic acid, and the depside atranorin. A close relative to this species is Parmotrema ultralucens, which contains the same cortical and medullary metabolites.
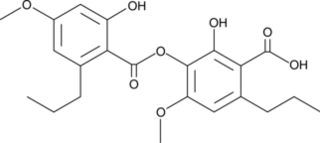
Sekikaic acid is an organic compound in the structural class of chemicals known as depsides. It is found in some lichens. First isolated from Ramalina sekika, it is a fairly common lichen product in the genera Ramalina and Cladonia. The species epithet of the powdery lichen Lepraria sekikaica refers to the presence of this substance—a rarity in genus Lepraria.
Henricus (Harrie) Johannes Maria Sipman is a Dutch lichenologist. He specialises in tropical and subtropical lichens, and has authored or co-authored more than 250 scientific publications. He was the curator of the lichen herbarium at the Berlin Botanical Garden and Botanical Museum from 1983 until his retirement in 2010.

Ramalina americana, commonly known as the sinewed ramalina, is a pale green fruticose lichen that is found across the Northern US Midwest, extending into Southern Canada and the Eastern Seaboard. It is characterized morphologically by the presence of pseudocyphellae, straight spores, and its unique chemical diversity.









