
A dye is a colored substance that chemically bonds to the substrate to which it is being applied. This distinguishes dyes from pigments which do not chemically bind to the material they color. Dye is generally applied in an aqueous solution and may require a mordant to improve the fastness of the dye on the fiber.

Haematoxylin or hematoxylin, also called natural black 1 or C.I. 75290, is a compound extracted from heartwood of the logwood tree with a chemical formula of C
16H
14O
6. This naturally derived dye has been used as a histologic stain, ink and as a dye in the textile and leather industry. As a dye, haematoxylin has been called Palo de Campeche, logwood extract, bluewood and blackwood. In histology, haematoxylin staining is commonly followed (counterstained), with eosin, when paired, this staining procedure is known as H&E staining, and is one of the most commonly used combinations in histology. In addition to its use in the H&E stain, haematoxylin is also a component of the Papanicolaou stain which is widely used in the study of cytology specimens.

Romanowsky staining, also known as Romanowsky–Giemsa staining, is a prototypical staining technique that was the forerunner of several distinct but similar stains widely used in hematology and cytopathology. Romanowsky-type stains are used to differentiate cells for microscopic examination in pathological specimens, especially blood and bone marrow films, and to detect parasites such as malaria within the blood. Stains that are related to or derived from the Romanowsky-type stains include Giemsa, Jenner, Wright, Field, May–Grünwald and Leishman stains. The staining technique is named after the Russian physician Dmitri Leonidovich Romanowsky (1861–1921), who was one of the first to recognize its potential for use as a blood stain.

Staining is a technique used to enhance contrast in samples, generally at the microscopic level. Stains and dyes are frequently used in histology, in cytology, and in the medical fields of histopathology, hematology, and cytopathology that focus on the study and diagnoses of diseases at the microscopic level. Stains may be used to define biological tissues, cell populations, or organelles within individual cells.

Methyl yellow, or C.I. 11020, is an organic compound with the formula C6H5N2C6H4N(CH3)2. It is an azo dye derived from dimethylaniline. It is a yellow solid. According to X-ray crystallography, the C14N3 core of the molecule is planar.

Hoechst stains are part of a family of blue fluorescent dyes used to stain DNA. These bis-benzimides were originally developed by Hoechst AG, which numbered all their compounds so that the dye Hoechst 33342 is the 33,342nd compound made by the company. There are three related Hoechst stains: Hoechst 33258, Hoechst 33342, and Hoechst 34580. The dyes Hoechst 33258 and Hoechst 33342 are the ones most commonly used and they have similar excitation–emission spectra.

Sudan I is an organic compound, typically classified as an azo dye. It is an intensely orange-red solid that is added to colourise waxes, oils, petrol, solvents, and polishes. Sudan I has also been adopted for colouring various foodstuffs, especially curry powder and chili powder, although the use of Sudan I in foods is now banned in many countries, because Sudan I, Sudan III, and Sudan IV have been classified as category 3 carcinogens by the International Agency for Research on Cancer. Sudan I is still used in some orange-coloured smoke formulations and as a colouring for cotton refuse used in chemistry experiments.

Nile blue is a stain used in biology and histology. It may be used with live or fixed cells, and imparts a blue colour to cell nuclei.
Nile red is a lipophilic stain. Nile red stains intracellular lipid droplets yellow. In most polar solvents, Nile red will not fluoresce; however, when in a lipid-rich environment, it can be intensely fluorescent, with varying colors from deep red to strong yellow-gold emission. The dye is highly solvatochromic and its emission and excitation wavelength both shift depending on solvent polarity and in polar media will hardly fluoresce at all.
Sudan IV (C24H20N4O) is a lysochrome (fat-soluble dye) diazo dye used for the staining of lipids, triglycerides and lipoproteins on frozen paraffin sections. It has the appearance of reddish brown crystals with melting point 199 °C and maximum absorption at 520(357) nm.
Trypan blue is an azo dye. It is a direct dye for cotton textiles. In biosciences, it is used as a vital stain to selectively colour dead tissues or cells blue.

Sudan III is a lysochrome diazo dye. It is structurally related to azobenzene.

Oil Red O (Solvent Red 27, Sudan Red 5B, C.I. 26125, C26H24N4O) is a lysochrome (fat-soluble dye) diazo dye used for staining of neutral triglycerides and lipids on frozen sections and some lipoproteins on paraffin sections. It has the appearance of a red powder with an absorbance maxima at 518 nanometers.

Sudan II (Solvent Orange 7, C.I. 12140, C18H16N2O) is a lysochrome (fat-soluble dye) azo dye used for staining of triglycerides in frozen sections, and some protein bound lipids and lipoproteins on paraffin sections. It has the appearance of red powder with melting point 156–158 °C and maximum absorption at 493(420) nm.

Hematein or haematein is an oxidized derivative of haematoxylin, used in staining. Haematein should not be confused with haematin, which is a brown to black iron-containing pigment formed by decomposition of haemoglobin. In the Colour Index, haematein is called haematine.

Hematoxylin and eosin stain is one of the principal tissue stains used in histology. It is the most widely used stain in medical diagnosis and is often the gold standard. For example, when a pathologist looks at a biopsy of a suspected cancer, the histological section is likely to be stained with H&E.
Sudan stains and Sudan dyes are synthetic organic compounds that are used as dyes for various plastics and are also used to stain sudanophilic biological samples, usually lipids. Sudan II, Sudan III, Sudan IV, Oil Red O, and Sudan Black B are important members of this class of compounds.

Sudan Red 7B, also known as Solvent Red 19, Ceres Red 7B, Fat Red 7B, Hexatype carmine B, Lacquer red V3B, Oil violet, Organol bordeaux B, Sudanrot 7B, Typogen carmine, and C.I. 26050, is a red diazo dye. Chemically it is N-ethyl-1-[[p-(phenylazo)phenyl]azo]-2-naphthalenamine. It is soluble in oils and insoluble in water.
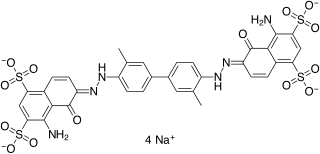
T-1824 or Evans blue, often incorrectly rendered as Evan's blue, is an azo dye that has a very high affinity for serum albumin. Because of this, it can be useful in physiology in estimating the proportion of body water contained in blood plasma. It fluoresces with excitation peaks at 470 and 540 nm and an emission peak at 680 nm.

Alcian blue is any member of a family of polyvalent basic dyes, of which the Alcian blue 8G has been historically the most common and the most reliable member. It is used to stain acidic polysaccharides such as glycosaminoglycans in cartilages and other body structures, some types of mucopolysaccharides, sialylated glycocalyx of cells etc. For many of these targets it is one of the most widely used cationic dyes for both light and electron microscopy. Use of alcian blue has historically been a popular staining method in histology especially for light microscopy in paraffin embedded sections and in semithin resin sections. The tissue parts that specifically stain by this dye become blue to bluish-green after staining and are called "Alcianophilic". Alcian blue staining can be combined with H&E staining, PAS staining and van Gieson staining methods. Alcian blue can be used to quantitate acidic glycans both in microspectrophotometric quantitation in solution or for staining glycoproteins in polyacrylamide gels or on western blots. Biochemists had used it to assay acid polysaccharides in urine since the 1960s for diagnosis of diseases like mucopolysaccharidosis but from 1970's, partly due to lack of availability of Alcian and partly due to length and tediousness of the procedure, alternative methods had to be developed e.g. Dimethyl methylene blue method.


















