
Ascomycota is a phylum of the kingdom Fungi that, together with the Basidiomycota, forms the subkingdom Dikarya. Its members are commonly known as the sac fungi or ascomycetes. It is the largest phylum of Fungi, with over 64,000 species. The defining feature of this fungal group is the "ascus", a microscopic sexual structure in which nonmotile spores, called ascospores, are formed. However, some species of Ascomycota are asexual and thus do not form asci or ascospores. Familiar examples of sac fungi include morels, truffles, brewers' and bakers' yeast, dead man's fingers, and cup fungi. The fungal symbionts in the majority of lichens such as Cladonia belong to the Ascomycota.
An ascocarp, or ascoma, is the fruiting body (sporocarp) of an ascomycete phylum fungus. It consists of very tightly interwoven hyphae and millions of embedded asci, each of which typically contains four to eight ascospores. Ascocarps are most commonly bowl-shaped (apothecia) but may take on a spherical or flask-like form that has a pore opening to release spores (perithecia) or no opening (cleistothecia).
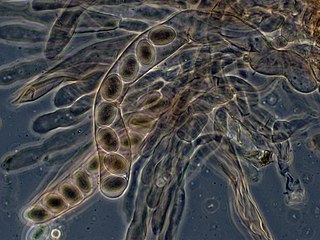
An ascus is the sexual spore-bearing cell produced in ascomycete fungi. Each ascus usually contains eight ascospores, produced by meiosis followed, in most species, by a mitotic cell division. However, asci in some genera or species can occur in numbers of one, two, four, or multiples of four. In a few cases, the ascospores can bud off conidia that may fill the asci with hundreds of conidia, or the ascospores may fragment, e.g. some Cordyceps, also filling the asci with smaller cells. Ascospores are nonmotile, usually single celled, but not infrequently may be coenocytic, and in some cases coenocytic in multiple planes. Mitotic divisions within the developing spores populate each resulting cell in septate ascospores with nuclei. The term ocular chamber, or oculus, refers to the epiplasm that is surrounded by the "bourrelet".

Neurospora crassa is a type of red bread mold of the phylum Ascomycota. The genus name, meaning 'nerve spore' in Greek, refers to the characteristic striations on the spores. The first published account of this fungus was from an infestation of French bakeries in 1843.
Heterothallic species have sexes that reside in different individuals. The term is applied particularly to distinguish heterothallic fungi, which require two compatible partners to produce sexual spores, from homothallic ones, which are capable of sexual reproduction from a single organism.

Neurospora is a genus of Ascomycete fungi. The genus name, meaning "nerve spore" refers to the characteristic striations on the spores that resemble axons.

Fungi are a diverse group of organisms that employ a huge variety of reproductive strategies, ranging from fully asexual to almost exclusively sexual species. Most species can reproduce both sexually and asexually, alternating between haploid and diploid forms. This contrasts with most multicellular eukaryotes such as mammals, where the adults are usually diploid and produce haploid gametes which combine to form the next generation. In fungi, both haploid and diploid forms can reproduce – haploid individuals can undergo asexual reproduction while diploid forms can produce gametes that combine to give rise to the next generation.

Venturia inaequalis is an ascomycete fungus that causes the apple scab disease.

The Sordariaceae are a family of perithecial fungi within the Sordariales order.

Dikarya is a subkingdom of Fungi that includes the divisions Ascomycota and Basidiomycota, both of which in general produce dikaryons, may be filamentous or unicellular, but are always without flagella. The Dikarya are most of the so-called "higher fungi", but also include many anamorphic species that would have been classified as molds in historical literature. Phylogenetically the two divisions regularly group together. In a 1998 publication, Thomas Cavalier-Smith referred to this group as the Neomycota.

Xylaria polymorpha, commonly known as dead man's fingers, is a cosmopolitan saprobic fungus. It is characterized by its elongated upright, clavate, or strap-like stromata poking up through the ground, much like fingers.
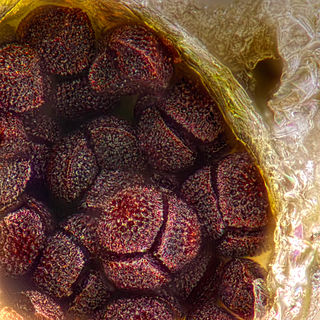
The tetrad is the four spores produced after meiosis of a yeast or other Ascomycota, Chlamydomonas or other alga, or a plant. After parent haploids mate, they produce diploids. Under appropriate environmental conditions, diploids sporulate and undergo meiosis. The meiotic products, spores, remain packaged in the parental cell body to produce the tetrad.

Sporormiella is a genus of fungi in the phylum Ascomycota whose species can be found worldwide, including the Arctic. It grows primarily on dung but also can be found in soil and plant debris. The exact number of species is debated and can range from 60 to 80 in total depending on the source. A majority of these species are coprophilous, however, there are a few that are endophytes and saprobic.
Sphaerotheca castagnei is a species of ascomycete fungus in the family Erysiphaceae. A plant pathogen, it causes a form of powdery mildew.

Chaetomium globosum is a well-known mesophilic member of the mold family Chaetomiaceae. It is a saprophytic fungus that primarily resides on plants, soil, straw, and dung. Endophytic C. globosum assists in cellulose decomposition of plant cells. They are found in habitats ranging from forest plants to mountain soils across various biomes. C. globosum colonies can also be found indoors and on wooden products.
Arcopilus aureus is a plant and soil fungus in the genus Arcopilus. It was first identified by A. H. Chivers in 1912, who named it Chaetomium aureum. It was later transferred to the genus Arcopilus by Wang and colleagues. The fungus has recently been recognized to have industrial use for the production of the metabolites resveratrol. and sclerotiorin Additionally, A. aureus has high lead tolerance and clearance, suggesting a potential role in environmental biotechnology.
Podospora appendiculata is a coprophilous fungus that is most commonly found in the dung of lagomorphs, such as hares and rabbits, in temperate to warm climates. A member of the division Ascomycota, P. appendiculata is characterized by ovoid, hair-studded perithecia which can bear a distinctive violaceous colouring and peridia which are coriaceous, or leathery, in texture. Podospora appendiculata has been shown to produce three compounds with antimicrobial properties.
Triangularia setosa is a member of the Ascomycota, and of the genus Triangularia. This genus is notable for its widespread appearance on the excrement of herbivores, and is therefore seen as a coprophilous fungus. The fungus itself is characteristically dark in colour and produces sac-like perithecium with a covering of hair. Its dispersion involves the ingestion, passage, and projectile ejection of spores. It has preference for colonizing the dung of lagomorphs, such as hares and rabbits.
Cercophora areolata is a member of the Ascomycota division, and is grouped into the Lasiosphaeriaceae family based on morphology. C. areolata is a coprophilous fungus that has been most recently isolated from porcupine dung. Defining features of C. areolata include: 1) ovoid-conical, glabrous ascomata, 2) black, carbonaceous, areolate peridium and 3) clavate-shaped, single-walled asci. From studies on C. areolata, this fungus produces multiple antifungal compounds, which inhibit other competitor fungi.
Protothelenella is a genus of fungi in the family Protothelenellaceae. It contains 11 species, some of which form lichens. Protothelenella species have a crustose thallus with spherical to pear-shaped, dark brown to blackish perithecia. Microscopic characteristics of the genus include bitunicate asci with an amyloid tholus, and ascospores that are colourless and contain multiple internal partitions. Some species grow on acidic substrates including rocks, soil, bryophytes, plant detritus or rotten wood. Other species are lichenicolous (lichen-dwelling), growing on species of Solorina, Peltigera, Pseudocyphellaria, or Cladonia.















