
Spectroscopy is the field of study that measures and interprets electromagnetic spectra. In narrower contexts, spectroscopy is the precise study of color as generalized from visible light to all bands of the electromagnetic spectrum.
Circular dichroism (CD) is dichroism involving circularly polarized light, i.e., the differential absorption of left- and right-handed light. Left-hand circular (LHC) and right-hand circular (RHC) polarized light represent two possible spin angular momentum states for a photon, and so circular dichroism is also referred to as dichroism for spin angular momentum. This phenomenon was discovered by Jean-Baptiste Biot, Augustin Fresnel, and Aimé Cotton in the first half of the 19th century. Circular dichroism and circular birefringence are manifestations of optical activity. It is exhibited in the absorption bands of optically active chiral molecules. CD spectroscopy has a wide range of applications in many different fields. Most notably, UV CD is used to investigate the secondary structure of proteins. UV/Vis CD is used to investigate charge-transfer transitions. Near-infrared CD is used to investigate geometric and electronic structure by probing metal d→d transitions. Vibrational circular dichroism, which uses light from the infrared energy region, is used for structural studies of small organic molecules, and most recently proteins and DNA.

Magnetic circular dichroism (MCD) is the differential absorption of left and right circularly polarized light, induced in a sample by a strong magnetic field oriented parallel to the direction of light propagation. MCD measurements can detect transitions which are too weak to be seen in conventional optical absorption spectra, and it can be used to distinguish between overlapping transitions. Paramagnetic systems are common analytes, as their near-degenerate magnetic sublevels provide strong MCD intensity that varies with both field strength and sample temperature. The MCD signal also provides insight into the symmetry of the electronic levels of the studied systems, such as metal ion sites.

A synchrotron light source is a source of electromagnetic radiation (EM) usually produced by a storage ring, for scientific and technical purposes. First observed in synchrotrons, synchrotron light is now produced by storage rings and other specialized particle accelerators, typically accelerating electrons. Once the high-energy electron beam has been generated, it is directed into auxiliary components such as bending magnets and insertion devices in storage rings and free electron lasers. These supply the strong magnetic fields perpendicular to the beam that are needed to stimulate the high energy electrons to emit photons.

Fluorescence spectroscopy is a type of electromagnetic spectroscopy that analyzes fluorescence from a sample. It involves using a beam of light, usually ultraviolet light, that excites the electrons in molecules of certain compounds and causes them to emit light; typically, but not necessarily, visible light. A complementary technique is absorption spectroscopy. In the special case of single molecule fluorescence spectroscopy, intensity fluctuations from the emitted light are measured from either single fluorophores, or pairs of fluorophores.

X-ray absorption fine structure (XAFS) is a specific structure observed in X-ray absorption spectroscopy (XAS). By analyzing the XAFS, information can be acquired on the local structure and on the unoccupied local electronic states.

Extended X-ray absorption fine structure (EXAFS), along with X-ray absorption near edge structure (XANES), is a subset of X-ray absorption spectroscopy (XAS). Like other absorption spectroscopies, XAS techniques follow Beer's law. The X-ray absorption coefficient of a material as a function of energy is obtained by directing X-rays of a narrow energy range at a sample, while recording the incident and transmitted x-ray intensity, as the incident x-ray energy is incremented.

Extreme ultraviolet radiation or high-energy ultraviolet radiation is electromagnetic radiation in the part of the electromagnetic spectrum spanning wavelengths shorter that the hydrogen Lyman-alpha line from 121 nm down to the X-ray band of 10 nm, and therefore having photons with energies from 10.26 eV up to 124.24 eV. EUV is naturally generated by the solar corona and artificially by plasma, high harmonic generation sources and synchrotron light sources. Since UVC extends to 100 nm, there is some overlap in the terms.
Multi-wavelength anomalous diffraction is a technique used in X-ray crystallography that facilitates the determination of the three-dimensional structure of biological macromolecules via solution of the phase problem.
Diffraction topography is a imaging technique based on Bragg diffraction. Diffraction topographic images ("topographies") record the intensity profile of a beam of X-rays diffracted by a crystal. A topography thus represents a two-dimensional spatial intensity mapping of reflected X-rays, i.e. the spatial fine structure of a Laue reflection. This intensity mapping reflects the distribution of scattering power inside the crystal; topographs therefore reveal the irregularities in a non-ideal crystal lattice. X-ray diffraction topography is one variant of X-ray imaging, making use of diffraction contrast rather than absorption contrast which is usually used in radiography and computed tomography (CT). Topography is exploited to a lesser extends with neutrons, and has similarities to dark field imaging in the electron microscope community.
Vibrational circular dichroism (VCD) is a spectroscopic technique which detects differences in attenuation of left and right circularly polarized light passing through a sample. It is the extension of circular dichroism spectroscopy into the infrared and near infrared ranges.

The European X-Ray Free-Electron Laser Facility is an X-ray research laser facility commissioned during 2017. The first laser pulses were produced in May 2017 and the facility started user operation in September 2017. The international project with twelve participating countries; nine shareholders at the time of commissioning, later joined by three other partners, is located in the German federal states of Hamburg and Schleswig-Holstein. A free-electron laser generates high-intensity electromagnetic radiation by accelerating electrons to relativistic speeds and directing them through special magnetic structures. The European XFEL is constructed such that the electrons produce X-ray light in synchronisation, resulting in high-intensity X-ray pulses with the properties of laser light and at intensities much brighter than those produced by conventional synchrotron light sources.
Single-wavelength anomalous diffraction (SAD) is a technique used in X-ray crystallography that facilitates the determination of the structure of proteins or other biological macromolecules by allowing the solution of the phase problem. In contrast to multi-wavelength anomalous diffraction (MAD), SAD uses a single dataset at a single appropriate wavelength.
The Protein Circular Dichroism Data Bank (PCDDB) is a database of circular dichroism and synchrotron radiation.
Electron magnetic circular dichroism (EMCD) is the EELS equivalent of XMCD.
Photoelectron photoion coincidence spectroscopy (PEPICO) is a combination of photoionization mass spectrometry and photoelectron spectroscopy. It is largely based on the photoelectric effect. Free molecules from a gas-phase sample are ionized by incident vacuum ultraviolet (VUV) radiation. In the ensuing photoionization, a cation and a photoelectron are formed for each sample molecule. The mass of the photoion is determined by time-of-flight mass spectrometry, whereas, in current setups, photoelectrons are typically detected by velocity map imaging. Electron times-of-flight are three orders of magnitude smaller than those of ions, which allows electron detection to be used as a time stamp for the ionization event, starting the clock for the ion time-of-flight analysis. In contrast with pulsed experiments, such as REMPI, in which the light pulse must act as the time stamp, this allows to use continuous light sources, e.g. a discharge lamp or a synchrotron light source. No more than several ion–electron pairs are present simultaneously in the instrument, and the electron–ion pairs belonging to a single photoionization event can be identified and detected in delayed coincidence.
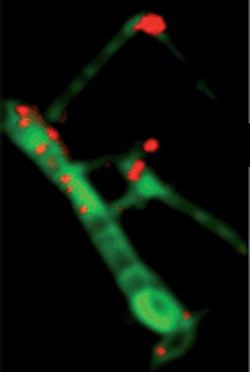
Scanning transmission X-ray microscopy (STXM) is a type of X-ray microscopy in which a zone plate focuses an X-ray beam onto a small spot, a sample is scanned in the focal plane of the zone plate and the transmitted X-ray intensity is recorded as a function of the sample position. A stroboscopic scheme is used where the excitation is the pump and the synchrotron X-ray flashes are the probe. X-ray microscopes work by exposing a film or charged coupled device detector to detect X-rays that pass through the specimen. The image formed is of a thin section of specimen. Newer X-ray microscopes use X-ray absorption spectroscopy to heterogeneous materials at high spatial resolution. The essence of the technique is a combination of spectromicroscopy, imaging with spectral sensitivity, and microspectroscopy, recording spectra from very small spots.
Serena DeBeer is an American chemist. She is currently a W3-Professor and the director at the Max Planck Institute for Chemical Energy Conversion in Muelheim an der Ruhr, Germany, where she heads the Department of Inorganic Spectroscopy. Her expertise lies in the application and development of X-ray based spectroscopic methods as probes of electronic structure in biological and chemical catalysis.

SOLARIS is the only synchrotron in Central-Eastern Europe. Built in Poland in 2015, under the auspices of the Jagiellonian University, it is located on the Campus of the 600th Anniversary of the Jagiellonian University Revival, in the southern part of Kraków. It is the central facility of the National Synchrotron Radiation Centre SOLARIS.
Bonnie Ann Wallace, FRSC is a British and American biophysicist and biochemist. She is a professor of molecular biophysics in the department of biological sciences, formerly the department of crystallography, at Birkbeck College, University of London, U.K.









