Related Research Articles

Hepatitis C is an infectious disease caused by the hepatitis C virus (HCV) that primarily affects the liver; it is a type of viral hepatitis. During the initial infection people often have mild or no symptoms. Occasionally a fever, dark urine, abdominal pain, and yellow tinged skin occurs. The virus persists in the liver in about 75% to 85% of those initially infected. Early on, chronic infection typically has no symptoms. Over many years however, it often leads to liver disease and occasionally cirrhosis. In some cases, those with cirrhosis will develop serious complications such as liver failure, liver cancer, or dilated blood vessels in the esophagus and stomach.

Blood transfusion is the process of transferring blood products into a person's circulation intravenously. Transfusions are used for various medical conditions to replace lost components of the blood. Early transfusions used whole blood, but modern medical practice commonly uses only components of the blood, such as red blood cells, white blood cells, plasma, clotting factors and platelets.
Hepatology is the branch of medicine that incorporates the study of liver, gallbladder, biliary tree, and pancreas as well as management of their disorders. Although traditionally considered a sub-specialty of gastroenterology, rapid expansion has led in some countries to doctors specializing solely on this area, who are called hepatologists.
Transfusion medicine is the branch of medicine that encompasses all aspects of the transfusion of blood and blood components including aspects related to hemovigilance. It includes issues of blood donation, immunohematology and other laboratory testing for transfusion-transmitted diseases, management and monitoring of clinical transfusion practices, patient blood management, therapeutic apheresis, stem cell collections, cellular therapy, and coagulation. Laboratory management and understanding of state and federal regulations related to blood products are also a large part of the field.
A blood-borne disease is a disease that can be spread through contamination by blood and other body fluids. Blood can contain pathogens of various types, chief among which are microorganisms, like bacteria and parasites, and non-living infectious agents such as viruses. Three blood-borne pathogens in particular, all viruses, are cited as of primary concern to health workers by the CDC-NIOSH: HIV, hepatitis B (HVB), & hepatitis C (HVC).

HIV tests are used to detect the presence of the human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), in serum, saliva, or urine. Such tests may detect antibodies, antigens, or RNA.

A blood donation occurs when a person voluntarily has blood drawn and used for transfusions and/or made into biopharmaceutical medications by a process called fractionation. Donation may be of whole blood, or of specific components directly (apheresis). Blood banks often participate in the collection process as well as the procedures that follow it.

Chiron Corporation was an American multinational biotechnology firm founded in 1981, based in Emeryville, California, that was acquired by Novartis on April 20, 2006. It had offices and facilities in eighteen countries on five continents. Chiron's business and research was in three main areas: biopharmaceuticals, vaccines, and blood testing. Chiron's vaccines and blood testing units were combined to form Novartis Vaccines and Diagnostics, while Chiron BioPharmaceuticals was integrated into Novartis Pharmaceuticals. In 2014, Novartis completed the sale of its blood transfusion diagnostics unit to Grifols and announced agreements for the sale of its vaccines unit to GlaxoSmithKline.

Sree Chitra Tirunal Institute for Medical Sciences & Technology (SCTIMST), Trivandrum, is an Institution of National Importance established by an Act of Parliament in 1980. It is under the aegis of Department of Science and Technology, Government of India, with an Institute Body and a Governing Body constituted as per the provisions of the Sree Chitra Tirunal Institute for Medical Sciences & Technology, Trivandrum, Act, 1980. The Institute presents a unique model by connecting the different strands of Biomedical Technology ,Clinical Medicine and Public Health to produce a seamless continuum of indisputable relevance to society.
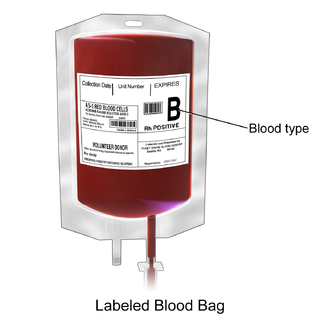
Packed red blood cells, also known as packed cells, are red blood cells that have been separated for blood transfusion. The packed cells are typically used in anemia that is either causing symptoms or when the hemoglobin is less than usually 70–80 g/L. In adults, one unit brings up hemoglobin levels by about 10 g/L. Repeated transfusions may be required in people receiving cancer chemotherapy or who have hemoglobin disorders. Cross-matching is typically required before the blood is given. It is given by injection into a vein.
Ortho Clinical Diagnostics is an in vitro diagnostics company that makes products and diagnostic equipment for blood testing. Ortho serves two primary industries in the medical field: clinical laboratories, by producing platforms and assays that test for a variety of diseases, conditions, and substances; and immunohematology, by providing the means to ensure blood transfusion recipients receive appropriate and compatible blood.
Johnson and Johnson acquired Eastman Kodak's Clinical Diagnostics Division in 1994, which was then merged with Ortho Diagnostic Systems in 1997. Ortho's global corporate offices are in Raritan, New Jersey, while their global research and development center is in Rochester, New York.

A medical laboratory or clinical laboratory is a laboratory where tests are conducted out on clinical specimens to obtain information about the health of a patient to aid in diagnosis, treatment, and prevention of disease. Clinical Medical laboratories are an example of applied science, as opposed to research laboratories that focus on basic science, such as found in some academic institutions.
A transfusion transmitted infection (TTI) is a virus, parasite, or other potential pathogen that can be transmitted in donated blood through a transfusion to a recipient. The term is usually limited to known pathogens, but also sometimes includes agents such as Simian foamy virus which are not known to cause disease.

The 2009 Gujarat hepatitis B outbreak was a cluster of hepatitis B cases that appeared in Modasa, northern Gujarat, India in 2009. Over 125 people were infected and up to 49 people died. Several doctors were investigated and arrested after the outbreaks.
Husaini Blood Bank (HBB) is a public health organization with its headquarter at Karachi and working for the welfare of the people of Pakistan via its sub offices and affiliated department/NGOs all over the country. HBB was founded in 1979 by Dr Hasan Ali Vajid with the establishment of Husaini Haematology and Oncology Trust inside his Clinic at Soldier Bazar Karachi through the support of Mr Hamid D. Habib, the chairman of Habib Trusts and started blood collection and donation at a very small scale.
Barcode technology in healthcare is the use of optical machine-readable representation of data in a hospital or healthcare setting.

AMRI Hospitals is a private hospital chain which is headquartered in the city of Kolkata, West Bengal, India. The company's head office is in Kolkata, West Bengal, with 3 units in Kolkata, 1 clinic in Kolkata and 1 unit Bhubaneshwar in the Indian State of Odisha. The hospital had also opened a health center in Dhaka for its Bangladeshi patients.
Infectious diseases within American correctional settings are a concern within the public health sector. The corrections population is susceptible to infectious diseases through exposure to blood and other bodily fluids, drug injection, poor health care, prison overcrowding, demographics, security issues, lack of community support for rehabilitation programs, and high-risk behaviors. The spread of infectious diseases, such as HIV and other sexually transmitted diseases, hepatitis C (HCV), hepatitis B (HBV), and tuberculosis, result largely from needle-sharing, drug use, and consensual and non-consensual sex among prisoners. HIV and hepatitis C need specific attention because of the specific public health concerns and issues they raise.
Blood donations in India are conducted by organisations and hospitals through blood donation camps. Donors can also visit blood banks in hospitals to donate blood. Efforts by the government and advocacy groups over the years have helped bridge the gap between demand and supply. The regulatory framework for blood donation and blood bank management rests with the Central Drugs Standard Control Organisation, while technical bodies like the National Blood Transfusion Council and National AIDS Control Organisation formulate guidelines and recommendations for transfusion medicine and blood bank management. Challenges persist with regards to regulation of blood banks and transfusion practices as the sector is largely fragmented with uneven distribution of blood banks and supply of blood in parts of the country. Donors are usually provided with refreshments after the procedure, which include glucose drinks, biscuits and fruits. Some organisations offer transportation facilities, as well as certificates or badges as gratitude.
References
- ↑ Shah, Mansi (2007). "Waiting for health care: a survey of a public hospital in Kolkata" (PDF). Centre for Civil Society. Archived from the original (PDF) on 13 August 2011. Retrieved 31 January 2012.
- ↑ "Archived copy" (PDF). Archived from the original (PDF) on 2016-03-09. Retrieved 2018-11-19.
{{cite web}}: CS1 maint: archived copy as title (link) - 1 2 "Defective blood-test kits in West Bengal" . Retrieved 2018-11-19.
- 1 2 3 4 5 6 7 "Unknown vs The State Of West Bengal on 14 November, 2014". indiankanoon.org. Retrieved 2018-11-18.
- 1 2 "Compensation Demanded For Bengal's Blood Scam Victims". Medindia. Retrieved 2018-11-18.
- 1 2 "Blood test kit probe heads to Hong Kong". www.telegraphindia.com. Retrieved 2018-11-19.
- 1 2 "Jute baron's bro held - Times of India". The Times of India. Retrieved 2018-11-19.
- ↑ "NACO supplying bogus HIV testing kits: WB". www.hindustantimes.com/. 2007-07-06. Retrieved 2018-11-19.