Xeroderma pigmentosum, complementation group C, also known as XPC, is a protein which in humans is encoded by the XPC gene. XPC is involved in the recognition of bulky DNA adducts in nucleotide excision repair. [4] It is located on chromosome 3. [5]
Xeroderma pigmentosum, complementation group C, also known as XPC, is a protein which in humans is encoded by the XPC gene. XPC is involved in the recognition of bulky DNA adducts in nucleotide excision repair. [4] It is located on chromosome 3. [5]
This gene encodes a component of the nucleotide excision repair (NER) pathway. There are multiple components involved in the NER pathway, including Xeroderma pigmentosum (XP) A-G and V, Cockayne syndrome (CS) A and B, and trichothiodystrophy (TTD) group A, etc. This component, XPC, plays an important role in the early steps of global genome NER, especially in damage recognition, open complex formation, and repair protein complex formation. [4]
The complex of XPC-RAD23B is the initial damage recognition factor in global genomic nucleotide excision repair (GG-NER). [6] XPC-RAD23B recognizes a wide variety of lesions that thermodynamically destabilize DNA duplexes, including UV-induced photoproducts (cyclopyrimidine dimers and 6-4 photoproducts ), adducts formed by environmental mutagens such as benzo[a]pyrene or various aromatic amines, certain oxidative endogenous lesions such as cyclopurines and adducts formed by cancer chemotherapeutic drugs such as cisplatin. The presence of XPC-RAD23B is required for assembly of the other core NER factors and progression through the NER pathway both in vitro and in vivo. [7] Although most studies have been performed with XPC-RAD23B, it is part of a trimeric complex with centrin-2, a calcium-binding protein of the calmodulin family. [7]
Mutations in this gene or some other NER components result in Xeroderma pigmentosum, a rare autosomal recessive disorder characterized by increased sensitivity to sunlight with the development of carcinomas at an early age. [4]
DNA damage appears to be the primary underlying cause of cancer, [8] and deficiencies in DNA repair genes likely underlie many forms of cancer. [9] [10] If DNA repair is deficient, DNA damage tends to accumulate. Such excess DNA damage may increase mutations due to error-prone translesion synthesis. Excess DNA damage may also increase epigenetic alterations due to errors during DNA repair. [11] [12] Such mutations and epigenetic alterations may give rise to cancer.
Reductions in expression of DNA repair genes (usually caused by epigenetic alterations such as promoter hypermethylation) are very common in cancers, and are ordinarily much more frequent than mutational defects in DNA repair genes in cancers.[ citation needed ] The table below shows that XPC expression was frequently epigenetically reduced in bladder cancer and also in non-small cell lung cancer, and also shows that XPC was more frequently reduced in the more advanced stages of these cancers.
| Cancer | Frequency | Ref. |
|---|---|---|
| Bladder cancer | 50% | [13] |
| Papillary urothelial neoplasm of low malignant potential | 35% | [13] |
| Low grade papillary bladder cancer | 42% | [13] |
| High grade papillary bladder cancer | 65% | [13] |
| Non-small cell lung cancer (NSCLC) | 70% | [14] |
| NSCLC Stage I | 62% | [14] |
| NSCLC Stages II-III | 77% | [14] |
While epigenetic hypermethylation of the promoter region of the XPC gene was shown to be associated with low expression of XPC, [13] another mode of epigenetic repression of XPC may also occur by over-expression of the microRNA miR-890. [15]
XPC (gene) has been shown to interact with ABCA1, [16] CETN2 [17] and XPB. [18]
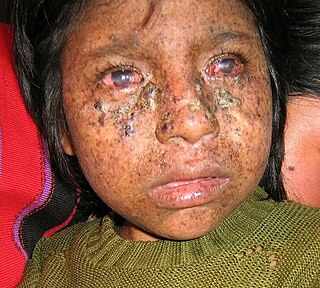
Xeroderma pigmentosum (XP) is a genetic disorder in which there is a decreased ability to repair DNA damage such as that caused by ultraviolet (UV) light. Symptoms may include a severe sunburn after only a few minutes in the sun, freckling in sun-exposed areas, dry skin and changes in skin pigmentation. Nervous system problems, such as hearing loss, poor coordination, loss of intellectual function and seizures, may also occur. Complications include a high risk of skin cancer, with about half having skin cancer by age 10 without preventative efforts, and cataracts. There may be a higher risk of other cancers such as brain cancers.

Nucleotide excision repair is a DNA repair mechanism. DNA damage occurs constantly because of chemicals, radiation and other mutagens. Three excision repair pathways exist to repair single stranded DNA damage: Nucleotide excision repair (NER), base excision repair (BER), and DNA mismatch repair (MMR). While the BER pathway can recognize specific non-bulky lesions in DNA, it can correct only damaged bases that are removed by specific glycosylases. Similarly, the MMR pathway only targets mismatched Watson-Crick base pairs.

XPB is an ATP-dependent DNA helicase in humans that is a part of the TFIIH transcription factor complex.
Richard D. Wood is an American molecular biologist specializing in research on DNA repair and mutation. He is known for pioneering studies on nucleotide excision repair (NER), particularly for reconstituting the minimum set of proteins involved in this process, identifying proliferating cell nuclear antigen (PCNA) as part of the NER complex and identifying mammalian repair polymerases.

ERCC2, or XPD is a protein involved in transcription-coupled nucleotide excision repair.
Transcription factor II H (TFIIH) is an important protein complex, having roles in transcription of various protein-coding genes and DNA nucleotide excision repair (NER) pathways. TFIIH first came to light in 1989 when general transcription factor-δ or basic transcription factor 2 was characterized as an indispensable transcription factor in vitro. This factor was also isolated from yeast and finally named TFIIH in 1992.

DNA excision repair protein ERCC-1 is a protein that in humans is encoded by the ERCC1 gene. Together with ERCC4, ERCC1 forms the ERCC1-XPF enzyme complex that participates in DNA repair and DNA recombination.

UV excision repair protein RAD23 homolog A is a protein that in humans is encoded by the RAD23A gene.

DNA damage-binding protein 2 is a protein that in humans is encoded by the DDB2 gene.

UV excision repair protein RAD23 homolog B is a protein that in humans is encoded by the RAD23B gene.

DNA repair protein complementing XP-A cells is a protein that in humans is encoded by the XPA gene.

DNA excision repair protein ERCC-6 is a protein that in humans is encoded by the ERCC6 gene. The ERCC6 gene is located on the long arm of chromosome 10 at position 11.23.

DNA repair protein complementing XP-G cells is a protein that in humans is encoded by the ERCC5 gene.
Centrin-2 is a protein that in humans is encoded by the CETN2 gene. It belongs to the centrin family of proteins.
ERCC4 is a protein designated as DNA repair endonuclease XPF that in humans is encoded by the ERCC4 gene. Together with ERCC1, ERCC4 forms the ERCC1-XPF enzyme complex that participates in DNA repair and DNA recombination.

DNA polymerase eta, is a protein that in humans is encoded by the POLH gene.

Trichothiodystrophy (TTD) is an autosomal recessive inherited disorder characterised by brittle hair and intellectual impairment. The word breaks down into tricho – "hair", thio – "sulphur", and dystrophy – "wasting away" or literally "bad nourishment". TTD is associated with a range of symptoms connected with organs of the ectoderm and neuroectoderm. TTD may be subclassified into four syndromes: Approximately half of all patients with trichothiodystrophy have photosensitivity, which divides the classification into syndromes with or without photosensitivity; BIDS and PBIDS, and IBIDS and PIBIDS. Modern covering usage is TTD-P (photosensitive), and TTD.
In molecular biology, the XPC binding domain is thought to play a role in DNA damage discrimination and in the enhancement of cell survival. They bind specifically and directly to the xeroderma pigmentosum group C protein (XPC) to initiate nucleotide excision repair (NER). Members of this entry adopt a structure consisting of four alpha helices, arranged in an array.
In molecular biology the protein domain XPG refers to, in this case, the N-terminus of XPG. The XPG protein can be corrected by a 133 kDa nuclear protein, XPGC. XPGC is an acidic protein that confers normal ultraviolet (UV) light resistance. It is a magnesium-dependent, single-strand DNA endonuclease that makes structure-specific endonucleolytic incisions in a DNA substrate containing a duplex region and single-stranded arms. XPGC cleaves one strand of the duplex at the border with the single-stranded region.
Progeroid syndromes (PS) are a group of rare genetic disorders that mimic physiological aging, making affected individuals appear to be older than they are. The term progeroid syndrome does not necessarily imply progeria, which is a specific type of progeroid syndrome.