Uracil-DNA glycosylase (also known as UNG or UDG) is an enzyme. Its most important function is to prevent mutagenesis by eliminating uracil from DNA molecules by cleaving the N-glycosidic bond and initiating the base-excision repair (BER) pathway.
Uracil-DNA glycosylase (also known as UNG or UDG) is an enzyme. Its most important function is to prevent mutagenesis by eliminating uracil from DNA molecules by cleaving the N-glycosidic bond and initiating the base-excision repair (BER) pathway.
The human gene encodes one of several uracil-DNA glycosylases. Alternative promoter usage and splicing of this gene leads to two different isoforms: the mitochondrial UNG1 and the nuclear UNG2. [5] One important function of uracil-DNA glycosylases is to prevent mutagenesis by eliminating uracil from DNA molecules by cleaving the N-glycosidic bond and initiating the base-excision repair (BER) pathway. Uracil bases occur from cytosine deamination or misincorporation of dUMP residues. After a mutation occurs, the mutagenic threat of uracil propagates through any subsequent DNA replication steps. [6] Once unzipped, mismatched guanine and uracil pairs are separated, and DNA polymerase inserts complementary bases to form a guanine-cytosine (GC) pair in one daughter strand and an adenine-uracil (AU) pair in the other. [7] Half of all progeny DNA derived from the mutated template inherit a shift from GC to AU at the mutation site. [7] UDG excises uracil in both AU and GU pairs to prevent propagation of the base mismatch to downstream transcription and translation processes. [7] With high efficiency and specificity, this glycosylase repairs 100–500 bases damaged daily in the human cell. [8] Human cells express five to six types of DNA glycosylases, all of which share a common mechanism of base eversion and excision as a means of DNA repair. [9]
UDG is made of a four-stranded parallel β-sheet surrounded by eight α-helices. [10] The active site comprises five highly conserved motifs that collectively catalyze glycosidic bond cleavage: [11] [12]
Glycosidic bond cleavage follows a “pinch-push-pull” mechanism using the five conserved motifs. [10]
Pinch: UDG scans DNA for uracil by nonspecifically binding to the strand and creating a kink in the backbone, thereby positioning the selected base for detection. The Pro-rich and Gly-Ser loops form polar contacts with the 3’ and 5’ phosphates flanking the examined base. [11] This compression of the DNA backbone, or “pinch,” allows for close contact between UDG and base of interest. [10]
Push: To fully assess the nucleotide identity, the intercalation loop penetrates, or pushes into, the DNA minor groove and induces a conformational change to flip the nucleotide out of the helix. [13] Backbone compression favors eversion of the now extrahelical nucleotide, which is positioned for recognition by the uracil-binding motif. [10] The coupling of intercalation and eversion helps compensate for the disruption of favorable base stacking interactions within the DNA helix. Leu272 fills the void left by the flipped nucleotide to create dispersion interactions with neighboring bases and restore stacking stability. [11]
Pull: Now accessible to the active site, the nucleotide interacts with the uracil binding motif. The active site shape complements the everted uracil structure, allowing for high substrate specificity. Purines are too large to fit in the active site, while unfavorable interactions with other pyrimidines discourage binding alternative substrates. [9] The side chain of Tyr147 interferes sterically with the thymine C5 methyl group, while a specific hydrogen bond between the uracil O2 carbonyl and Gln144 discriminates against a cytosine substrate, which lacks the necessary carbonyl. [9] Once uracil is recognized, cleavage of the glycosidic bond proceeds according to the mechanism below.



The position of the residues that activate the water nucleophile and protonate the uracil leaving group are widely debated, though the most commonly followed mechanism employs the water activating loop detailed in the enzyme structure. [12] [14] Regardless of position, the identities of the aspartic acid and histidine residues are consistent across catalytic studies. [10] [11] [12] [14] [15]
Uracil N-glycosylase (UNG) is utilized to eliminate carryover polymerase chain reaction (PCR) products in PCR. This method modifies PCR products such that in a new reaction, any residual products from previous PCR amplifications will be digested and prevented from amplifying, but the true DNA templates will be unaffected. [16] PCR synthesizes abundant amplification products each round, but contamination of further rounds of PCR with trace amounts of these products, called carry-over contamination, yields false positive results. Carry-over contamination from some previous PCR can be a significant problem, due both to the abundance of PCR products, and to the ideal structure of the contaminant material for re-amplification. However carry-over contamination can be controlled by the following two steps: (i) incorporating dUTP in all PCR products (by substituting dUTP for dTTP, or by incorporating uracil during synthesis of primers; and (ii) treating all subsequent fully preassembled starting reactions with uracil DNA glycosylase (UDG), followed by thermal inactivation of UDG. UDG cleaves the uracil base from the phosphodiester backbone of uracil-containing DNA, but has no effect on natural (i.e., thymine-containing) DNA. The resulting apyrimidinic sites block replication by DNA polymerases, and are very labile to acid/base hydrolysis. Because UDG does not react with dTTP, and is also inactivated by heat denaturation prior to the actual PCR, carry-over contamination of PCRs can be controlled effectively if the contaminants contain uracils in place of thymines. [6]
Uracil N-glycosylase was also used in a study to detect evidence of ongoing low-level metabolic activity and DNA repair in ancient bacteria. [17] Long-term survival of bacteria can occur either through endospore formation (in which the bacterium enters total dormancy, with no metabolic activity at all taking place, and, thus, no DNA repair), or else through reduction of metabolic activity to a very low rate, just sufficient to carry out ongoing DNA repair and prevent the depletion of other unstable molecules (such as ATP), in which the microbe is able to repair damage to its DNA but also continues to slowly consume nutrients. [17] DNA sequences from bacteria in permafrost were amplified using PCR. One series of runs amplified the DNA sequences as-is (to detect all live bacterial DNA in the samples), while the other series looked specifically for DNA that had been undergoing ongoing repair; to do this, the DNA was treated with UNG to remove uracils. This prevented amplification of unrepaired DNA in two ways: firstly, the abasic sites generated by the removal of uracils prevented the DNA polymerase used in PCR from proceeding past the site of damage, while these abasic sites also directly weakened the DNA and made it more likely to fragment upon heating. [17] In this way, the researchers were able to show evidence of ongoing DNA repair in high-GC Gram-positive bacteria up to 600,000 years old. [17]
Uracil N glycosylase has also been used in a method for cloning of PCR amplified DNA fragments. In this method the primers used in PCR are synthesized with uracil residues instead of thymine. When these primers are incorporated into PCR amplified fragments the primer sequence becomes susceptible to digestion with Uracil N Glycosylase and produce 3' protruding ends that can be annealed to an appropriately prepared vector DNA. The resulting chimeric molecules can be transformed into competent cells with high efficiency, without the need for in vitro ligation. [18]
Uracil-DNA glycosylase has been shown to interact with RPA2. [19]
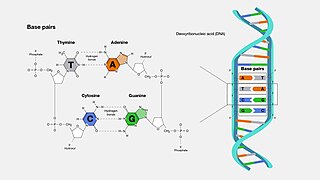
A base pair (bp) is a fundamental unit of double-stranded nucleic acids consisting of two nucleobases bound to each other by hydrogen bonds. They form the building blocks of the DNA double helix and contribute to the folded structure of both DNA and RNA. Dictated by specific hydrogen bonding patterns, "Watson–Crick" base pairs allow the DNA helix to maintain a regular helical structure that is subtly dependent on its nucleotide sequence. The complementary nature of this based-paired structure provides a redundant copy of the genetic information encoded within each strand of DNA. The regular structure and data redundancy provided by the DNA double helix make DNA well suited to the storage of genetic information, while base-pairing between DNA and incoming nucleotides provides the mechanism through which DNA polymerase replicates DNA and RNA polymerase transcribes DNA into RNA. Many DNA-binding proteins can recognize specific base-pairing patterns that identify particular regulatory regions of genes.
Deamination is the removal of an amino group from a molecule. Enzymes that catalyse this reaction are called deaminases.
Protein engineering is the process of developing useful or valuable proteins through the design and production of unnatural polypeptides, often by altering amino acid sequences found in nature. It is a young discipline, with much research taking place into the understanding of protein folding and recognition for protein design principles. It has been used to improve the function of many enzymes for industrial catalysis. It is also a product and services market, with an estimated value of $168 billion by 2017.

DNA synthesis is the natural or artificial creation of deoxyribonucleic acid (DNA) molecules. DNA is a macromolecule made up of nucleotide units, which are linked by covalent bonds and hydrogen bonds, in a repeating structure. DNA synthesis occurs when these nucleotide units are joined to form DNA; this can occur artificially or naturally. Nucleotide units are made up of a nitrogenous base, pentose sugar (deoxyribose) and phosphate group. Each unit is joined when a covalent bond forms between its phosphate group and the pentose sugar of the next nucleotide, forming a sugar-phosphate backbone. DNA is a complementary, double stranded structure as specific base pairing occurs naturally when hydrogen bonds form between the nucleotide bases.

In biochemistry, a nuclease is an enzyme capable of cleaving the phosphodiester bonds that link nucleotides together to form nucleic acids. Nucleases variously affect single and double stranded breaks in their target molecules. In living organisms, they are essential machinery for many aspects of DNA repair. Defects in certain nucleases can cause genetic instability or immunodeficiency. Nucleases are also extensively used in molecular cloning.
DNA glycosylases are a family of enzymes involved in base excision repair, classified under EC number EC 3.2.2. Base excision repair is the mechanism by which damaged bases in DNA are removed and replaced. DNA glycosylases catalyze the first step of this process. They remove the damaged nitrogenous base while leaving the sugar-phosphate backbone intact, creating an apurinic/apyrimidinic site, commonly referred to as an AP site. This is accomplished by flipping the damaged base out of the double helix followed by cleavage of the N-glycosidic bond.

Nucleotide excision repair is a DNA repair mechanism. DNA damage occurs constantly because of chemicals, radiation and other mutagens. Three excision repair pathways exist to repair single stranded DNA damage: Nucleotide excision repair (NER), base excision repair (BER), and DNA mismatch repair (MMR). While the BER pathway can recognize specific non-bulky lesions in DNA, it can correct only damaged bases that are removed by specific glycosylases. Similarly, the MMR pathway only targets mismatched Watson-Crick base pairs.

In biochemistry and molecular genetics, an AP site, also known as an abasic site, is a location in DNA that has neither a purine nor a pyrimidine base, either spontaneously or due to DNA damage. It has been estimated that under physiological conditions 10,000 apurinic sites and 500 apyrimidinic may be generated in a cell daily.
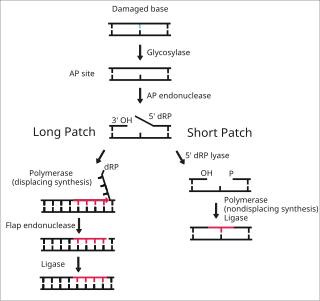
Base excision repair (BER) is a cellular mechanism, studied in the fields of biochemistry and genetics, that repairs damaged DNA throughout the cell cycle. It is responsible primarily for removing small, non-helix-distorting base lesions from the genome. The related nucleotide excision repair pathway repairs bulky helix-distorting lesions. BER is important for removing damaged bases that could otherwise cause mutations by mispairing or lead to breaks in DNA during replication. BER is initiated by DNA glycosylases, which recognize and remove specific damaged or inappropriate bases, forming AP sites. These are then cleaved by an AP endonuclease. The resulting single-strand break can then be processed by either short-patch or long-patch BER.

Pyrimidine dimers represent molecular lesions originating from thymine or cytosine bases within DNA, resulting from photochemical reactions. These lesions, commonly linked to direct DNA damage, are induced by ultraviolet light (UV), particularly UVC, result in the formation of covalent bonds between adjacent nitrogenous bases along the nucleotide chain near their carbon–carbon double bonds, the photo-coupled dimers are fluorescent. Such dimerization, which can also occur in double-stranded RNA (dsRNA) involving uracil or cytosine, leads to the creation of cyclobutane pyrimidine dimers (CPDs) and 6–4 photoproducts. These pre-mutagenic lesions modify the DNA helix structure, resulting in abnormal non-canonical base pairing and, consequently, adjacent thymines or cytosines in DNA will form a cyclobutane ring when joined together and cause a distortion in the DNA. This distortion prevents DNA replication and transcription mechanisms beyond the dimerization site.

Cyclin-O is a protein that in humans is encoded by the CCNO gene.

G/T mismatch-specific thymine DNA glycosylase is an enzyme that in humans is encoded by the TDG gene. Several bacterial proteins have strong sequence homology with this protein.

DNA-3-methyladenine glycosylase also known as 3-alkyladenine DNA glycosylase (AAG) or N-methylpurine DNA glycosylase (MPG) is an enzyme that in humans is encoded by the MPG gene.

Single-strand selective monofunctional uracil DNA glycosylase is an enzyme that in humans is encoded by the SMUG1 gene. SMUG1 is a glycosylase that removes uracil from single- and double-stranded DNA in nuclear chromatin, thus contributing to base excision repair.
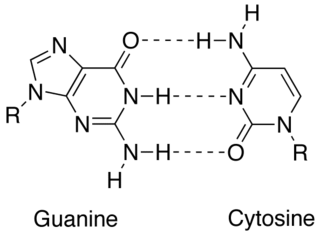
In molecular biology, complementarity describes a relationship between two structures each following the lock-and-key principle. In nature complementarity is the base principle of DNA replication and transcription as it is a property shared between two DNA or RNA sequences, such that when they are aligned antiparallel to each other, the nucleotide bases at each position in the sequences will be complementary, much like looking in the mirror and seeing the reverse of things. This complementary base pairing allows cells to copy information from one generation to another and even find and repair damage to the information stored in the sequences.

In molecular biology, the H2TH domain is a DNA-binding domain found in DNA glycosylase/AP lyase enzymes, which are involved in base excision repair of DNA damaged by oxidation or by mutagenic agents. Most damage to bases in DNA is repaired by the base excision repair pathway. These enzymes are primarily from bacteria, and have both DNA glycosylase activity EC 3.2.2.- and AP lyase activity EC 4.2.99.18. Examples include formamidopyrimidine-DNA glycosylases and endonuclease VIII (Nei).
DNA-deoxyinosine glycosylase is an enzyme with systematic name DNA-deoxyinosine deoxyribohydrolase. This enzyme is involved in DNA damage repair and targets hypoxanthine bases.
Double-stranded uracil-DNA glycosylase is an enzyme with systematic name uracil-double-stranded DNA deoxyribohydrolase (uracil-releasing). This enzyme catalyses a specific chemical reaction: it hydrolyses mismatched double-stranded DNA and polynucleotides, releasing free uracil.
Pwo polymerase is a thermostable DNA polymerase used for the polymerase chain reaction. The abbreviation stands for Pyrococcus woesei, a thermophilic archaeon, from which this polymerase was isolated. This polymerase breaks when reaching erroneous uracil in DNA from the chain extension and, through this readahead function, fewer defective DNA clones are synthesized. It is used much less than the usual Taq or Pfu polymerases. This DNA polymerase, similar to other DNA polymerases from Archaebacteria is sensitive to Uracil residues in DNA and is strongly inhibited by dUTP or uracil residues in DNA. Other polymerases in this class are Pfu, Vent, Deep Vent and Pfx. The inhibition of this class of thermostable DNA polymerases limit their use in some applications of PCR, i.e. use of dUTP for prevention of carryover contamination as well as application involving dU containing primers such as ligase free cloning methods or site directed mutagenesis using UNG.

DNA base flipping, or nucleotide flipping, is a mechanism in which a single nucleotide base, or nucleobase, is rotated outside the nucleic acid double helix. This occurs when a nucleic acid-processing enzyme needs access to the base to perform work on it, such as its excision for replacement with another base during DNA repair. It was first observed in 1994 using X-ray crystallography in a methyltransferase enzyme catalyzing methylation of a cytosine base in DNA. Since then, it has been shown to be used by different enzymes in many biological processes such as DNA methylation, various DNA repair mechanisms, and DNA replication. It can also occur in RNA double helices or in the DNA:RNA intermediates formed during RNA transcription.