Eucalyptol is a monoterpenoid colorless liquid, and a bicyclic ether. It has a fresh camphor-like odor and a spicy, cooling taste. It is insoluble in water, but miscible with organic solvents. Eucalyptol makes up about 70–90% of eucalyptus oil. Eucalyptol forms crystalline adducts with hydrohalic acids, o-cresol, resorcinol, and phosphoric acid. Formation of these adducts is useful for purification.

Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (Quercus ilex) and Norway spruce (Picea abies). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring.
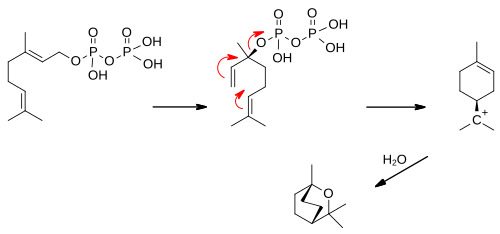
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ, as well as dehydrodolichol diphosphate.

In enzymology, bornyl diphosphate synthase (BPPS) (EC 5.5.1.8) is an enzyme that catalyzes the chemical reaction
The enzyme (4S)-limonene synthase catalyzes the chemical reaction
In enzymology, a pinene synthase is an enzyme that catalyzes the chemical reaction
The enzyme (R)-limonene synthase (EC 4.2.3.20) catalyzes the reversible chemical reaction
The enzyme sabinene-hydrate synthase (EC 4.2.3.11) catalyzes the chemical reaction
β-Phellandrene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase . This enzyme catalyses the following chemical reaction
(+)-Car-3-ene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (+)-car-3-ene-forming]. This enzyme catalyses the following chemical reaction
(–)-Sabinene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (–)-sabinene-forming]. This enzyme catalyses the following chemical reaction
(+)-Sabinene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (+)-sabinene-forming]. This enzyme catalyses the following chemical reaction
Terpinolene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase . This enzyme catalyses the following chemical reaction
γ-Terpinene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase . This enzyme catalyses the following chemical reaction
(+)-camphene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (+)-camphene-forming]. This enzyme catalyses the following chemical reaction
(–)-camphene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (–)-camphene-forming]. This enzyme catalyses the following chemical reaction
(–)-α-Pinene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (–)-α-pinene-forming]. This enzyme catalyses the following chemical reaction
(–)-β-Pinene synthase (EC 4.2.3.120, β-geraniolene synthase, (–)-(1S,5S)-pinene synthase, geranyldiphosphate diphosphate lyase (pinene forming)) is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (–)-β-pinene-forming]. This enzyme catalyses the following chemical reaction
(+)-α-pinene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [cyclizing, (+)-α-pinene-forming]. This enzyme catalyses the following chemical reaction
(+)-β-Pinene synthase is an enzyme with systematic name geranyl-diphosphate diphosphate-lyase [(+)-β-pinene-forming]. This enzyme catalyses the following chemical reaction