Related Research Articles

Sialic acids are a class of alpha-keto acid sugars with a nine-carbon backbone. The term "sialic acid" was first introduced by Swedish biochemist Gunnar Blix in 1952. The most common member of this group is N-acetylneuraminic acid found in animals and some prokaryotes.
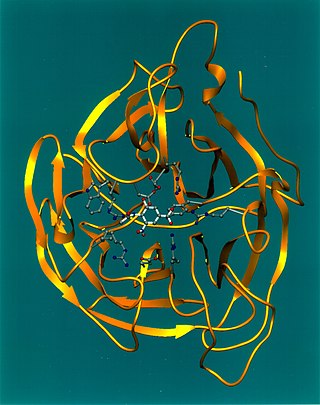
Exo-α-sialidase is a glycoside hydrolase that cleaves the glycosidic linkages of neuraminic acids:

Mucolipidosis type I is an inherited lysosomal storage disease that results from a deficiency of the enzyme alpha-N -acetyl neuraminidase (sialidase). The lack of this enzyme results in an abnormal accumulation of complex carbohydrates known as mucopolysaccharides, and of fatty substances known as mucolipids. Both of these substances accumulate in bodily tissues.
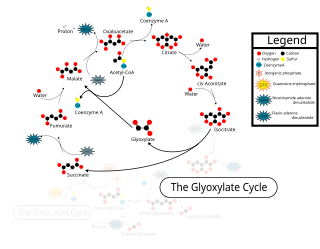
The glyoxylate cycle, a variation of the tricarboxylic acid cycle, is an anabolic pathway occurring in plants, bacteria, protists, and fungi. The glyoxylate cycle centers on the conversion of acetyl-CoA to succinate for the synthesis of carbohydrates. In microorganisms, the glyoxylate cycle allows cells to use two carbons, such as acetate, to satisfy cellular carbon requirements when simple sugars such as glucose or fructose are not available. The cycle is generally assumed to be absent in animals, with the exception of nematodes at the early stages of embryogenesis. In recent years, however, the detection of malate synthase (MS) and isocitrate lyase (ICL), key enzymes involved in the glyoxylate cycle, in some animal tissue has raised questions regarding the evolutionary relationship of enzymes in bacteria and animals and suggests that animals encode alternative enzymes of the cycle that differ in function from known MS and ICL in non-metazoan species.

The enzyme cystathionine γ-lyase (EC 4.4.1.1, CTH or CSE; also cystathionase; systematic name L-cystathionine cysteine-lyase (deaminating; 2-oxobutanoate-forming)) breaks down cystathionine into cysteine, 2-oxobutanoate (α-ketobutyrate), and ammonia:
In enzymology, an ascopyrone tautomerase is an enzyme that catalyzes the chemical reaction

Cystathionine beta-lyase, also commonly referred to as CBL or β-cystathionase, is an enzyme that primarily catalyzes the following α,β-elimination reaction

The enzyme N-acetylneuraminate lyase catalyzes the chemical reaction
The enzyme 1,5-anhydro-D-fructose dehydratase (EC 4.2.1.111) catalyzes the chemical reaction
The enzyme pectate disaccharide-lyase catalyzes the following process:
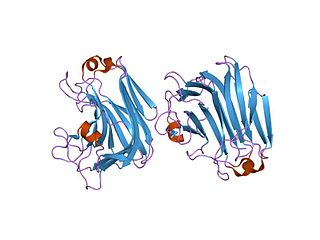
The enzyme mannuronate-specific alginate lyase catalyzes the degradation of alginate into various monosaccharide and polysaccharide products:

In enzymology, a malate synthase (EC 2.3.3.9) is an enzyme that catalyzes the chemical reaction
In enzymology, a N-acylneuraminate-9-phosphate synthase (EC 2.5.1.57) is an enzyme that catalyzes the chemical reaction

Sialidase-4 is an enzyme that in humans is encoded by the NEU4 gene.
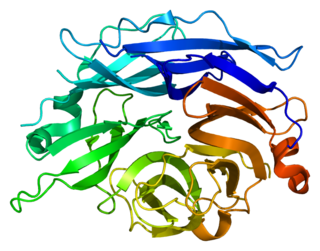
Sialidase-2 is an enzyme that in humans is encoded by the NEU2 gene.
Endo-α-sialidase is an enzyme with systematic name polysialoside (2→8)-α-sialosylhydrolase. It catalyses the following chemical reaction:
N-acetylmuramic acid 6-phosphate etherase (EC 4.2.1.126, MurNAc-6-P etherase, MurQ) is an enzyme with systematic name (R)-lactate hydro-lyase (adding N-acetyl-D-glucosamine 6-phosphate; N-acetylmuramate 6-phosphate-forming). This enzyme catalyses the following chemical reaction
The enzyme exo-(1→4)-α-D-glucan lyase (EC 4.2.2.13, α-(1→4)-glucan 1,5-anhydro-D-fructose eliminase, α-1,4-glucan exo-lyase, α-1,4-glucan lyase, GLase) is an enzyme with systematic name (1→4)-α-D-glucan exo-4-lyase (1,5-anhydro-D-fructose-forming). This enzyme catalyses the following chemical reaction

4-Hydroxy-tetrahydrodipicolinate synthase (EC 4.3.3.7, dihydrodipicolinate synthase, dihydropicolinate synthetase, dihydrodipicolinic acid synthase, L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing), dapA (gene)) is an enzyme with the systematic name L-aspartate-4-semialdehyde hydro-lyase (adding pyruvate and cyclizing; (4S)-4-hydroxy-2,3,4,5-tetrahydro-(2S)-dipicolinate-forming). This enzyme catalyses the following chemical reaction
Neuraminidase inhibitors inhibit enzymatic activity of the enzyme neuraminidase (sialidase). These type of inhibitors have been introduced as anti-influenza drugs as they prevent the virus from exiting infected cells and thus stop further spreading of the virus. Neuraminidase inhibitors for human neuraminidase (hNEU) have the potential to be useful drugs as the enzyme plays a role in several signaling pathways in cells and is implicated in diseases such as diabetes and cancer.
References
- Li YT, Nakagawa H, Ross SA, Hansson GC, Li SC (1990). "A novel sialidase which releases 2,7-anhydro-α-N-acetylneuraminic acid from sialoglycoconjugates". J. Biol. Chem. 265 (35): 21629–33. PMID 2254319.