| aspartate 4-decarboxylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
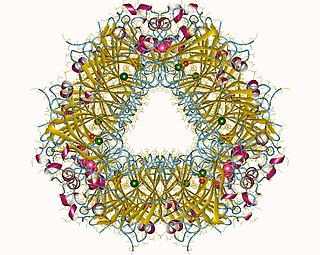
 Aspartate beta-decarboxylase dodekamer, Comamonas testosteroni | |||||||||
| Identifiers | |||||||||
| EC no. | 4.1.1.12 | ||||||||
| CAS no. | 9024-57-1 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
In enzymology, an aspartate 4-decarboxylase (EC 4.1.1.12) is an enzyme that catalyzes the chemical reaction
- L-aspartate L-alanine + CO2
Hence, this enzyme has one substrate, L-aspartate, and two products, L-alanine and CO2. This reaction is the basis of the industrial synthesis of L-alanine. [1]
This enzyme belongs to the family of lyases, specifically the carboxy-lyases, which cleave carbon-carbon bonds. The systematic name of this enzyme class is L-aspartate 4-carboxy-lyase (L-alanine-forming). Other names in common use include desulfinase, aminomalonic decarboxylase, aspartate beta-decarboxylase, aspartate omega-decarboxylase, aspartic omega-decarboxylase, aspartic beta-decarboxylase, L-aspartate beta-decarboxylase, cysteine sulfinic desulfinase, L-cysteine sulfinate acid desulfinase, and L-aspartate 4-carboxy-lyase. This enzyme participates in alanine and aspartate metabolism and cysteine metabolism. It employs one cofactor, pyridoxal phosphate.