
Cellular respiration is the process by which biological fuels are oxidised in the presence of an inorganic electron acceptor, such as oxygen, to drive the bulk production of adenosine triphosphate (ATP), which contains energy. Cellular respiration may be described as a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into ATP, and then release waste products.
In biochemistry, an oxidoreductase is an enzyme that catalyzes the transfer of electrons from one molecule, the reductant, also called the electron donor, to another, the oxidant, also called the electron acceptor. This group of enzymes usually utilizes NADP+ or NAD+ as cofactors. Transmembrane oxidoreductases create electron transport chains in bacteria, chloroplasts and mitochondria, including respiratory complexes I, II and III. Some others can associate with biological membranes as peripheral membrane proteins or be anchored to the membranes through a single transmembrane helix.

Biological carbon fixation or сarbon assimilation is the process by which inorganic carbon is converted to organic compounds by living organisms. The compounds are then used to store energy and as structure for other biomolecules. Carbon is primarily fixed through photosynthesis, but some organisms use a process called chemosynthesis in the absence of sunlight.
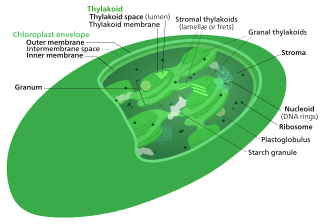
The Calvin cycle,light-independent reactions, bio synthetic phase,dark reactions, or photosynthetic carbon reduction (PCR) cycle of photosynthesis is a series of chemical reactions that convert carbon dioxide and hydrogen-carrier compounds into glucose. The Calvin cycle is present in all photosynthetic eukaryotes and also many photosynthetic bacteria. In plants, these reactions occur in the stroma, the fluid-filled region of a chloroplast outside the thylakoid membranes. These reactions take the products of light-dependent reactions and perform further chemical processes on them. The Calvin cycle uses the chemical energy of ATP and reducing power of NADPH from the light dependent reactions to produce sugars for the plant to use. These substrates are used in a series of reduction-oxidation reactions to produce sugars in a step-wise process; there is no direct reaction that converts several molecules of CO2 to a sugar. There are three phases to the light-independent reactions, collectively called the Calvin cycle: carboxylation, reduction reactions, and ribulose 1,5-bisphosphate (RuBP) regeneration.

Fructose-bisphosphate aldolase, often just aldolase, is an enzyme catalyzing a reversible reaction that splits the aldol, fructose 1,6-bisphosphate, into the triose phosphates dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate (G3P). Aldolase can also produce DHAP from other (3S,4R)-ketose 1-phosphates such as fructose 1-phosphate and sedoheptulose 1,7-bisphosphate. Gluconeogenesis and the Calvin cycle, which are anabolic pathways, use the reverse reaction. Glycolysis, a catabolic pathway, uses the forward reaction. Aldolase is divided into two classes by mechanism.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.
In enzymology, a carbonyl reductase (NADPH) (EC 1.1.1.184) is an enzyme that catalyzes the chemical reaction
In enzymology, a D-xylose 1-dehydrogenase (NADP+) (EC 1.1.1.179) is an enzyme that catalyzes the chemical reaction
In enzymology, a (3S,4R)-3,4-dihydroxycyclohexa-1,5-diene-1,4-dicarboxylate dehydrogenase (EC 1.3.1.53) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-hydroxybenzoyl-CoA reductase (EC 1.3.7.9) is an enzyme found in some bacteria and archaea that catalyzes the chemical reaction
In enzymology, an enoyl-[acyl-carrier-protein] reductase (NADPH, A-specific) (EC 1.3.1.39) is an enzyme that catalyzes the chemical reaction
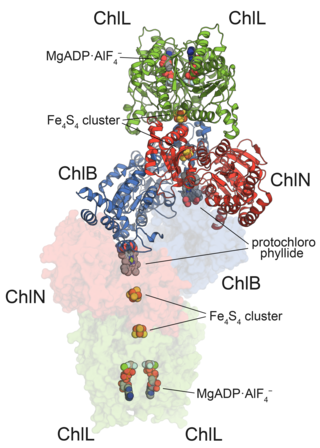
In enzymology, protochlorophyllide reductases (POR) are enzymes that catalyze the conversion from protochlorophyllide to chlorophyllide a. They are oxidoreductases participating in the biosynthetic pathway to chlorophylls.
In enzymology, a glyceraldehyde-3-phosphate dehydrogenase (ferredoxin) (EC 1.2.7.6) is an enzyme that catalyzes the chemical reaction

In enzymology, a saccharopine dehydrogenase (NADP+, L-glutamate-forming) (EC 1.5.1.10) is an enzyme that catalyzes the chemical reaction
In enzymology, a saccharopine dehydrogenase (NADP+, L-lysine-forming) (EC 1.5.1.8) is an enzyme that catalyzes the chemical reaction
The enzyme cyclohexa-1,5-dienecarbonyl-CoA hydratase (EC 4.2.1.100) catalyzes the chemical reaction
Bisphosphate may refer to:

Chlorophyllide a and Chlorophyllide b are the biosynthetic precursors of chlorophyll a and chlorophyll b respectively. Their propionic acid groups are converted to phytyl esters by the enzyme chlorophyll synthase in the final step of the pathway. Thus the main interest in these chemical compounds has been in the study of chlorophyll biosynthesis in plants, algae and cyanobacteria. Chlorophyllide a is also an intermediate in the biosynthesis of bacteriochlorophylls.

Chlorophyllide a reductase (EC 1.3.7.15), also known as COR, is an enzyme with systematic name bacteriochlorophyllide-a:ferredoxin 7,8-oxidoreductase. It catalyses the following chemical reaction
3-Hydroxybenzoyl-CoA is a molecule formed by condensing the thiol group of coenzyme A (CoA) with the carboxylic acid group of 3-hydroxybenzoic acid. Stable in acidic conditions, it is a tetraprotic acid due to the pyrophosphate and phosphate groups included. It derives from a benzoyl-CoA and a 3-hydroxybenzoic acid. In organisms such as plants, this can be formed using the 3-hydroxybenzoate—CoA ligase enzyme. This uses ATP, 3-hydroxybenzoate, and CoA as substrates.








