
Sarcoscypha is a genus of ascomycete fungus and a type genus of the family Sarcoscyphaceae. Species of Sarcoscypha are present in Europe, North America and tropical Asia. They are characterised by a cup-shaped apothecium which is often brightly coloured. They have had a range of popular uses, one of which was as a table decoration. Some members of the family such as S. coccinea and the - according to new knowledge - more common S. austriaca in western Europe and United States have bright scarlet apothecia which have given them familiar names such as the scarlet cup fungus and scarlet elf cap.

The fungus Cochliobolus sativus is the teleomorph of Bipolaris sorokiniana (anamorph) which is the causal agent of a wide variety of cereal diseases. The pathogen can infect and cause disease on roots, leaf and stem, and head tissue. C. sativus is extremely rare in nature and thus it is the asexual or anamorphic stage which causes infections. The two most common diseases caused by B. sorokiniana are spot blotch and common root rot, mainly on wheat and barley crops.

Cochliobolus carbonum is one of more than 40 species of filamentous ascomycetes belonging to the genus Cochliobolus. This pathogen has a worldwide distribution, with reports from Australia, Brazil, Cambodia, Canada, China, Congo, Denmark, Egypt, India, Kenya, New Zealand, Nigeria, Solomon Islands, and the United States. Cochliobolus carbonum is one of the most aggressive members of this genus infecting sorghum, corn and apple. As one of the most devastating pathogens of sweet corn, C. carbonum causes Northern leaf spot and ear rot disease while the asexual stage causes Helminthosporium corn leaf spot. Cochliobolus carbonum is pathogenic to all organs of the corn plant including root, stalk, ear, kernel, and sheath. However, symptoms of infection show distinct manifestations in different plant parts: whole plant - seedling blight affects the whole plant, leaf discoloration and mycelial growth, black fungal spores and lesions appear on inflorescences and glumes, and grain covered with very dark brown to black mycelium which gives a characteristic charcoal appearance due to the production of conidia.
Farrowia is a genus of fungi within the Chaetomiaceae family.

Chlamydosauromyces punctatus is the sole species in the monotypic genus of fungi, Chlamydosauromyces in the family, Onygenaceae. It was found in the skin shed from frilled lizard. This fungus is mesophilic and digests hair. It reproduces both sexually and asexually. The fungus has so far not been reported to be pathogenic.
Chaetomium cupreum is a fungus in the family Chaetomiaceae. It is able to decay in manufactured cellulosic materials, and is known to antagonize a wide range of soil microorganisms. This species is component of the biocontrol agent, Ketomium, a commercial biofungicide. It has also been investigated for use in the production of natural dyes. Chaetomium cupreum is mesophilic and known to occur in harsh environments and can rapidly colonize organic substrates in soil. Laboratory cultures of C. cupreum can be propagated on a range of common growth media including potato dextrose at ambient or higher than ambient temperature producing cottony white colonies with a reddish reverse.

Aphanoascus fulvescens is a mould fungus that behaves as a keratinophilic saprotroph and belongs to the Ascomycota. It is readily isolated from soil and dung containing keratin-rich tissues that have been separated from their animal hosts. This organism, distributed worldwide, is most commonly found in areas of temperate climate, in keeping with its optimal growth temperature of 28 °C (82 °F). While A. fulvescens is recognized as a geophilic fungal species, it is also a facultative opportunistic pathogen. Although it is not a dermatophyte, A. fulvescens has occasionally been shown to cause onychomycosis infections in humans. Its recognition in the laboratory is clinically important for correct diagnosis and treatment of human dermal infections.
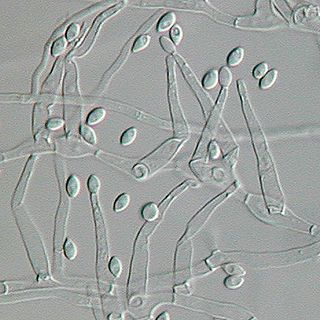
Paecilomyces variotii, also known by the name Byssochlamys spectabilis for the sexual state, is a common environmental mold from the Phylum Ascomycota. It is widespread in the environment and can be found in composts, soils and wood, as well es a common environmental contaminant in indoor air and carpet dust. Ascospores of the sexual state of P. variotii are strongly heat-resistant. As such the fungus is a common contaminant of heat-treated foods and juices. Paecilomyces variotii has been associated with a number of infective diseases of humans and animals.

Chaetomium globosum is a well-known mesophilic member of the mold family Chaetomiaceae. It is a saprophytic fungus that primarily resides on plants, soil, straw, and dung. Endophytic C. globosum assists in cellulose decomposition of plant cells. They are found in habitats ranging from forest plants to mountain soils across various biomes. C. globosum colonies can also be found indoors and on wooden products.
Thielavia subthermophila is a ubiquitous, filamentous fungus that is a member of the phylum Ascomycota and order Sordariales. Known to be found on plants of arid environments, it is an endophyte with thermophilic properties, and possesses dense, pigmented mycelium. Thielavia subthermophila has rarely been identified as a human pathogen, with a small number of clinical cases including ocular and brain infections. For treatment, antifungal drugs such as amphotericin B have been used topically or intravenously, depending upon the condition.
Aspergillus unguis is a species of fungus in the genus Aspergillus, and the asexual state (anamorph) of Emericella unguis. Aspergillus unguis is a filamentous soil-borne fungus found on decomposing plant matter and other moist substrates including with building materials and household dust. Aspergillus unguis occurs mainly in tropical and subtropical soils but has also been isolated from various marine and aquatic habitats. The species was first isolated in 1935 by Weill and L. Gaudin. Historically, A. unguis was assigned to the A. nidulans group, a common group of soil-borne fungi due to the resemblance of its ascospores and cleistothecia to those of Emericella nidulans. Aspergillus unguis is distinctive, however, in possessing spicular hyphae. A number of synonyms have been collapsed into this species, including Sterigmatocystis unguis, Aspergillus laokiashanensis and Aspergillus mellinus.

Keratinophyton durum is a keratinophilic fungus, that grows on keratin found in decomposing or shed animal hair and bird feathers. Various studies conducted in Canada, Japan, India, Spain, Poland, Ivory Coast and Iraq have isolated this fungus from decomposing animal hair and bird feathers using SDA and hair-bait technique. Presence of fungus in soil sediments and their ability to decompose hairs make them a potential human pathogen.
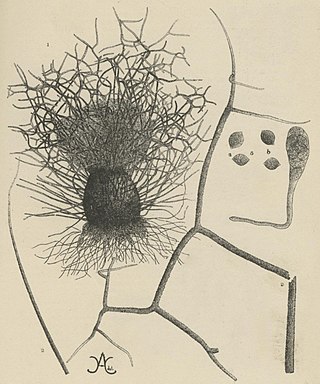
Chaetomium elatum is a very common and widely distributed saprotrophic fungus of the Chaetomiaceae family of molds which has been found to grow on many different substances all over the world. It was first established by Gustav Kunze after he observed it growing on dead leaves. Its defining features that distinguish it from other Chaetomium species are its extremely coarse terminal hairs and the lemon-shaped morphology of its ascospores. It produces many metabolites with potential biotechnology uses including one with promise against the rice blast disease fungus, Magnaporthe grisea. It shows very little pathogenic ability causing confirmed disease in only a few plant species.
Collariella bostrychodes is a fungal decomposer of lignin and carbohydrate in the family Chaetomiaceae commonly found in soil and dung. The fungus is distinguished by a darkened collar-like ostiole around the ostiolar pore, giving the fungus its name. The fungus is highly variable in shape and form, giving raise to the belief that there are two subclades in the species. The ascospores range from lemon-shaped to nearly spherical with slightly pointed ends. It can grow to be pale green and later turn pale bluish grey or olivaceous with age. The fungus produces the toxic secondary metabolite, chaetochromin.
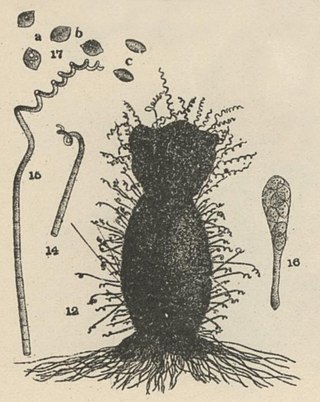
Chaetomium subspirale is a fungus from the phylum Ascomycota. It was described by A. H. Chivers in 1912 in America. The species has sexual fruiting bodies that are ornamented with characteristic, coiled hairs giving it a wooly appearance. C. subspirale colonies are brown, which the characteristic hairs are also responsible for. It is commonly found in various soil and dung samples. C. subspirale produces the mycotoxin, oxaspirodion, which inhibits inducible TNF-a expression and inhibits the activation of the transcription factor NF-kappaB.
Microascus manginii is a filamentous fungal species in the genus Microascus. It produces both sexual (teleomorph) and asexual (anamorph) reproductive stages known as M. manginii and Scopulariopsis candida, respectively. Several synonyms appear in the literature because of taxonomic revisions and re-isolation of the species by different researchers. M. manginii is saprotrophic and commonly inhabits soil, indoor environments and decaying plant material. It is distinguishable from closely related species by its light colored and heart-shaped ascospores used for sexual reproduction. Scopulariopsis candida has been identified as the cause of some invasive infections, often in immunocompromised hosts, but is not considered a common human pathogen. There is concern about amphotericin B resistance in S. candida.

Myxotrichum chartarum is a psychrophilic and cellulolytic fungus first discovered in Germany by Gustav Kunze in 1823. Its classification has changed many times over its history to better reflect the information available at the time. Currently, M. chartarum is known to be an ascomycete surrounded by a gymnothecium composed of ornate spines and releases asexual ascospores. The presence of cellulolytic processes are common in fungi within the family Myxotrichaceae. M. chartarum is one of many Myxotrichum species known to degrade paper and paper products. Evidence of M. chartarum "red spot" mold formation, especially on old books, can be found globally. As a result, this fungal species and other cellulolytic molds are endangering old works of art and books. Currently, there is no evidence that suggests that species within the family Myxotrichaceae are pathogenic.
Cephalotheca foveolata is a species of fungus. It is rarely opportunistic and generally manifests as a minor subcutaneous infection.
Cercophora areolata is a member of the Ascomycota division, and is grouped into the Lasiosphaeriaceae family based on morphology. C. areolata is a coprophilous fungus that has been most recently isolated from porcupine dung. Defining features of C. areolata include: 1) ovoid-conical, glabrous ascomata, 2) black, carbonaceous, areolate peridium and 3) clavate-shaped, single-walled asci. From studies on C. areolata, this fungus produces multiple antifungal compounds, which inhibit other competitor fungi.
Oidiodendron cereale is a species of ascomycetes fungi in the order Helotiales. This fungus is found globally in temperate climates where average summer temperatures are below 25 °C, but there have been scattered reports from tropical and subtropical environments. It is predominantly found in soil, but little is known regarding their ecological roles in nature. However, an enzymatic study from Agriculture Canada showed that O. cereale can break down a variety of plant, fungal, and animal based substrates found in soil, which may have beneficial effects for plants. On rare occasions, this fungus is found on human skin and hair. There has been one reported case of O. cereale infection in 1969, causing Neurodermitis Nuchae.










