
The cell cycle, or cell-division cycle, is the sequential series of events that take place in a cell that causes it to divide into two daughter cells. These events include the growth of the cell, duplication of its DNA and some of its organelles, and subsequently the partitioning of its cytoplasm, chromosomes and other components into two daughter cells in a process called cell division.
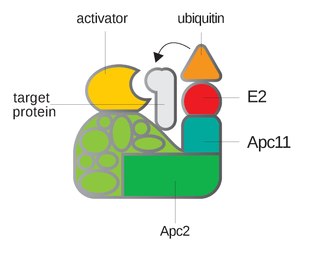
Anaphase-promoting complex is an E3 ubiquitin ligase that marks target cell cycle proteins for degradation by the 26S proteasome. The APC/C is a large complex of 11–13 subunit proteins, including a cullin (Apc2) and RING (Apc11) subunit much like SCF. Other parts of the APC/C have unknown functions but are highly conserved.

Cyclin-dependent kinases (CDKs) are a predominant group of serine/threonine protein kinases involved in the regulation of the cell cycle and its progression, ensuring the integrity and functionality of cellular machinery. These regulatory enzymes play a crucial role in the regulation of eukaryotic cell cycle and transcription, as well as DNA repair, metabolism, and epigenetic regulation, in response to several extracellular and intracellular signals. They are present in all known eukaryotes, and their regulatory function in the cell cycle has been evolutionarily conserved. The catalytic activities of CDKs are regulated by interactions with CDK inhibitors (CKIs) and regulatory subunits known as cyclins. Cyclins have no enzymatic activity themselves, but they become active once they bind to CDKs. Without cyclin, CDK is less active than in the cyclin-CDK heterodimer complex. CDKs phosphorylate proteins on serine (S) or threonine (T) residues. The specificity of CDKs for their substrates is defined by the S/T-P-X-K/R sequence, where S/T is the phosphorylation site, P is proline, X is any amino acid, and the sequence ends with lysine (K) or arginine (R). This motif ensures CDKs accurately target and modify proteins, crucial for regulating cell cycle and other functions. Deregulation of the CDK activity is linked to various pathologies, including cancer, neurodegenerative diseases, and stroke.
A cyclin-dependent kinase complex is a protein complex formed by the association of an inactive catalytic subunit of a protein kinase, cyclin-dependent kinase (CDK), with a regulatory subunit, cyclin. Once cyclin-dependent kinases bind to cyclin, the formed complex is in an activated state. Substrate specificity of the activated complex is mainly established by the associated cyclin within the complex. Activity of CDKCs is controlled by phosphorylation of target proteins, as well as binding of inhibitory proteins.

The restriction point (R), also known as the Start or G1/S checkpoint, is a cell cycle checkpoint in the G1 phase of the animal cell cycle at which the cell becomes "committed" to the cell cycle, and after which extracellular signals are no longer required to stimulate proliferation. The defining biochemical feature of the restriction point is the activation of G1/S- and S-phase cyclin-CDK complexes, which in turn phosphorylate proteins that initiate DNA replication, centrosome duplication, and other early cell cycle events. It is one of three main cell cycle checkpoints, the other two being the G2-M DNA damage checkpoint and the spindle checkpoint.

Cell cycle checkpoints are control mechanisms in the eukaryotic cell cycle which ensure its proper progression. Each checkpoint serves as a potential termination point along the cell cycle, during which the conditions of the cell are assessed, with progression through the various phases of the cell cycle occurring only when favorable conditions are met. There are many checkpoints in the cell cycle, but the three major ones are: the G1 checkpoint, also known as the Start or restriction checkpoint or Major Checkpoint; the G2/M checkpoint; and the metaphase-to-anaphase transition, also known as the spindle checkpoint. Progression through these checkpoints is largely determined by the activation of cyclin-dependent kinases by regulatory protein subunits called cyclins, different forms of which are produced at each stage of the cell cycle to control the specific events that occur therein.
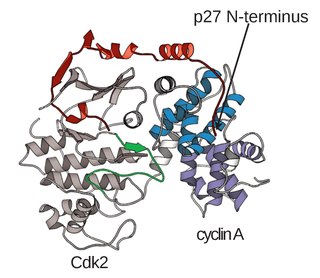
Cyclin A is a member of the cyclin family, a group of proteins that function in regulating progression through the cell cycle. The stages that a cell passes through that culminate in its division and replication are collectively known as the cell cycle Since the successful division and replication of a cell is essential for its survival, the cell cycle is tightly regulated by several components to ensure the efficient and error-free progression through the cell cycle. One such regulatory component is cyclin A which plays a role in the regulation of two different cell cycle stages.

Cyclin D is a member of the cyclin protein family that is involved in regulating cell cycle progression. The synthesis of cyclin D is initiated during G1 and drives the G1/S phase transition. Cyclin D protein is anywhere from 155 to 477 amino acids in length.
Transcription factor II H (TFIIH) is an important protein complex, having roles in transcription of various protein-coding genes and DNA nucleotide excision repair (NER) pathways. TFIIH first came to light in 1989 when general transcription factor-δ or basic transcription factor 2 was characterized as an indispensable transcription factor in vitro. This factor was also isolated from yeast and finally named TFIIH in 1992.
CDK7 is a cyclin-dependent kinase shown to be not easily classified. CDK7 is both a CDK-activating kinase (CAK) and a component of the general transcription factor TFIIH.

A cyclin-dependent kinase inhibitor protein(also known as CKIs, CDIs, or CDKIs) is a protein that inhibits the enzyme cyclin-dependent kinase (CDK) and Cyclin activity by stopping the cell cycle if there are unfavorable conditions, therefore, acting as tumor suppressors. Cell cycle progression is stopped by Cyclin-dependent kinase inhibitor protein at the G1 phase. CKIs are vital proteins within the control system that point out whether the processes of DNA synthesis, mitosis, and cytokines control one another. When a malfunction hinders the successful completion of DNA synthesis in the G1 phase, it triggers a signal that delays or halts the progression to the S phase. Cyclin-dependent kinase inhibitor proteins are essential in the regulation of the cell cycle. If cell mutations surpass the cell cycle checkpoints during cell cycle regulation, it can result in various types of cancer.

Cyclin-dependent kinase 1 also known as CDK1 or cell division cycle protein 2 homolog is a highly conserved protein that functions as a serine/threonine protein kinase, and is a key player in cell cycle regulation. It has been highly studied in the budding yeast S. cerevisiae, and the fission yeast S. pombe, where it is encoded by genes cdc28 and cdc2, respectively. With its cyclin partners, Cdk1 forms complexes that phosphorylate a variety of target substrates ; phosphorylation of these proteins leads to cell cycle progression.

Cyclin-dependent kinase 7, or cell division protein kinase 7, is an enzyme that in humans is encoded by the CDK7 gene.

CDK-activating kinase assembly factor MAT1 is an enzyme that in humans is encoded by the MNAT1 gene.

Cyclin-H is a protein that in humans is encoded by the CCNH gene.

General transcription factor IIH subunit 1 is a protein that in humans is encoded by the GTF2H1 gene.
RNA polymerase II holoenzyme is a form of eukaryotic RNA polymerase II that is recruited to the promoters of protein-coding genes in living cells. It consists of RNA polymerase II, a subset of general transcription factors, and regulatory proteins known as SRB proteins.

Pho4 is a protein with a basic helix-loop-helix (bHLH) transcription factor. It is found in S. cerevisiae and other yeasts. It functions as a transcription factor to regulate phosphate responsive genes located in yeast cells. The Pho4 protein homodimer is able to do this by binding to DNA sequences containing the bHLH binding site 5'-CACGTG-3'. This sequence is found in the promoters of genes up-regulated in response to phosphate availability such as the PHO5 gene.

In cell biology, eukaryotes possess a regulatory system that ensures that DNA replication occurs only once per cell cycle.

The Cyclin E/Cdk2 complex is a structure composed of two proteins, cyclin E and cyclin-dependent kinase 2 (Cdk2). Similar to other cyclin/Cdk complexes, the cyclin E/Cdk2 dimer plays a crucial role in regulating the cell cycle, with this specific complex peaking in activity during the G1/S transition. Once the cyclin and Cdk subunits join together, the complex gets activated, allowing it to phosphorylate and bind to downstream proteins to ultimately promote cell cycle progression. Although cyclin E can bind to other Cdk proteins, its primary binding partner is Cdk2, and the majority of cyclin E activity occurs when it exists as the cyclin E/Cdk2 complex.

















