Related Research Articles

Gangrene is a type of tissue death caused by a lack of blood supply. Symptoms may include a change in skin color to red or black, numbness, swelling, pain, skin breakdown, and coolness. The feet and hands are most commonly affected. If the gangrene is caused by an infectious agent, it may present with a fever or sepsis.

An ulcer is a sore on the skin or a mucous membrane, accompanied by the disintegration of tissue. Ulcers can result in complete loss of the epidermis and often portions of the dermis and even subcutaneous fat. Ulcers are most common on the skin of the lower extremities and in the gastrointestinal tract. An ulcer that appears on the skin is often visible as an inflamed tissue with an area of reddened skin. A skin ulcer is often visible in the event of exposure to heat or cold, irritation, or a problem with blood circulation.

Necrotizing fasciitis (NF), also known as flesh-eating disease, is a bacterial infection that results in the death of parts of the body's soft tissue. It is a severe disease of sudden onset that spreads rapidly. Symptoms usually include red or purple skin in the affected area, severe pain, fever, and vomiting. The most commonly affected areas are the limbs and perineum.

A wound is any disruption of or damage to living tissue, such as skin, mucous membranes, or organs. Wounds can either be the sudden result of direct trauma, or can develop slowly over time due to underlying disease processes such as diabetes mellitus, venous/arterial insufficiency, or immunologic disease. Wounds can vary greatly in their appearance depending on wound location, injury mechanism, depth of injury, timing of onset, and wound sterility, among other factors. Treatment strategies for wounds will vary based on the classification of the wound, therefore it is essential that wounds be thoroughly evaluated by a healthcare professional for proper management. In normal physiology, all wounds will undergo a series of steps collectively known as the wound healing process, which include hemostasis, inflammation, proliferation, and tissue remodeling. Age, tissue oxygenation, stress, underlying medical conditions, and certain medications are just a few of the many factors known to affect the rate of wound healing.
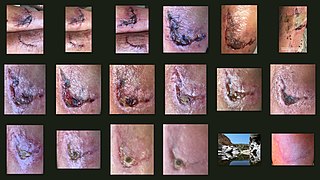
Wound healing refers to a living organism's replacement of destroyed or damaged tissue by newly produced tissue.

Pressure ulcers, also known as pressure sores, bed sores or pressure injuries, are localised damage to the skin and/or underlying tissue that usually occur over a bony prominence as a result of usually long-term pressure, or pressure in combination with shear or friction. The most common sites are the skin overlying the sacrum, coccyx, heels, and hips, though other sites can be affected, such as the elbows, knees, ankles, back of shoulders, or the back of the cranium.
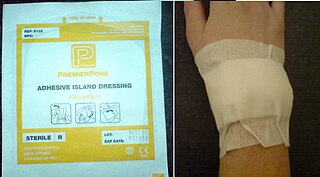
A dressing or compress is a piece of material such as a pad applied to a wound to promote healing and protect the wound from further harm. A dressing is designed to be in direct contact with the wound, as distinguished from a bandage, which is most often used to hold a dressing in place. Modern dressings are sterile.

Maggot therapy is a type of biotherapy involving the introduction of live, disinfected maggots into non-healing skin and soft-tissue wounds of a human or other animal for the purpose of cleaning out the necrotic (dead) tissue within a wound (debridement), and disinfection.

Venous ulcer is defined by the American Venous Forum as "a full-thickness defect of skin, most frequently in the ankle region, that fails to heal spontaneously and is sustained by chronic venous disease, based on venous duplex ultrasound testing." Venous ulcers are wounds that are thought to occur due to improper functioning of venous valves, usually of the legs. They are an important cause of chronic wounds, affecting 1% of the population. Venous ulcers develop mostly along the medial distal leg, and can be painful with negative effects on quality of life.
The history of wound care spans from prehistory to modern medicine. Wounds naturally heal by themselves, but hunter-gatherers would have noticed several factors and certain herbal remedies would speed up or assist the process, especially if it was grievous. In ancient history, this was followed by the realisation of the necessity of hygiene and the halting of bleeding, where wound dressing techniques and surgery developed. Eventually the germ theory of disease also assisted in improving wound care.

Negative-pressure wound therapy (NPWT), also known as a vacuum assisted closure (VAC), is a therapeutic technique using a suction pump, tubing, and a dressing to remove excess exudate and promote healing in acute or chronic wounds and second- and third-degree burns. The therapy involves the controlled application of sub-atmospheric pressure to the local wound environment using a sealed wound dressing connected to a vacuum pump. The use of this technique in wound management started in the 1990s and this technique is often recommended for treatment of a range of wounds including dehisced surgical wounds, closed surgical wounds, open abdominal wounds, open fractures, pressure injuries or pressure ulcers, diabetic foot ulcers, venous insufficiency ulcers, some types of skin grafts, burns, sternal wounds. It may also be considered after a clean surgery in a person who is obese.

Calciphylaxis, also known as calcific uremic arteriolopathy (CUA) or “Grey Scale”, is a rare syndrome characterized by painful skin lesions. The pathogenesis of calciphylaxis is unclear but believed to involve calcification of the small blood vessels located within the fatty tissue and deeper layers of the skin, blood clots, and eventual death of skin cells due to lack of blood flow. It is seen mostly in people with end-stage kidney disease but can occur in the earlier stages of chronic kidney disease and rarely in people with normally functioning kidneys. Calciphylaxis is a rare but serious disease, believed to affect 1-4% of all dialysis patients. It results in chronic non-healing wounds and indicates poor prognosis, with typical life expectancy of less than one year.
A hydrocolloid dressing is an opaque or transparent dressing for wounds. A hydrocolloid dressing is biodegradable, breathable, and depending on the dressing selected, may adhere to the skin, so no separate taping is needed.

Wound licking is an instinctive response in humans and many other animals to cover an injury or second degree burn with saliva. Dogs, cats, small rodents, horses, and primates all lick wounds. Saliva contains tissue factor which promotes the blood clotting mechanism. The enzyme lysozyme is found in many tissues and is known to attack the cell walls of many gram-positive bacteria, aiding in defense against infection. Tears are also beneficial to wounds due to the lysozyme enzyme. However, there are also infection risks due to bacteria in the mouth.
Wound bed preparation (WBP) is a systematic approach to wound management by identifying and removing barriers to healing. The concept was originally developed in plastic surgery. It includes wound assessment, debridement, moisture balance, bacterial balance, and wound cleaning.
Chronic wound pain is a condition described as unremitting, disabling, and recalcitrant pain experienced by individuals with various types of chronic wounds. Chronic wounds such as venous leg ulcers, arterial ulcers, diabetic foot ulcers, pressure ulcers, and malignant wounds can have an enormous impact on an individual’s quality of life with pain being one of the most distressing symptoms.

Diabetic foot ulcer is a breakdown of the skin and sometimes deeper tissues of the foot that leads to sore formation. It may occur due to a variety of mechanisms. It is thought to occur due to abnormal pressure or mechanical stress chronically applied to the foot, usually with concomitant predisposing conditions such as peripheral sensory neuropathy, peripheral motor neuropathy, autonomic neuropathy or peripheral arterial disease. It is a major complication of diabetes mellitus, and it is a type of diabetic foot disease. Secondary complications to the ulcer, such as infection of the skin or subcutaneous tissue, bone infection, gangrene or sepsis are possible, often leading to amputation.
Chronic limb threatening ischemia (CLTI), also known as critical limb ischemia (CLI), is an advanced stage of peripheral artery disease (PAD). It is defined as ischemic rest pain, arterial insufficiency ulcers, and gangrene. The latter two conditions are jointly referred to as tissue loss, reflecting the development of surface damage to the limb tissue due to the most severe stage of ischemia. Compared to the other manifestation of PAD, intermittent claudication, CLI has a negative prognosis within a year after the initial diagnosis, with 1-year amputation rates of approximately 12% and mortality of 50% at 5 years and 70% at 10 years.
The periwound is tissue surrounding a wound. Periwound area is traditionally limited to 4 cm outside the wound's edge but can extend beyond this limit if outward damage to the skin is present. Periwound assessment is an important step of wound assessment before wound treatment is prescribed.
Wound assessment is a component of wound management. As far as may be practical, the assessment is to be accomplished before prescribing any treatment plan. The objective is to collect information about the patient and about the wound, that may be relevant to planning and implementing the treatment.
References
- ↑ Mustoe T (March 17–18, 2005). "Dermal ulcer healing: Advances in understanding" (PDF). Tissue repair and ulcer/wound healing: molecular mechanisms, therapeutic targets and future directions. Paris, France: EUROCONFERENCES. Archived from the original (PDF) on October 27, 2005.
- 1 2 3 4 5 6 7 8 9 10 11 12 Snyder RJ (2005). "Treatment of nonhealing ulcers with allografts". Clinics in Dermatology. 23 (4): 388–95. doi:10.1016/j.clindermatol.2004.07.020. PMID 16023934.
- 1 2 3 4 Taylor JE, Laity PR, Hicks J, Wong SS, Norris K, Khunkamchoo P, et al. (October 2005). "Extent of iron pick-up in deforoxamine-coupled polyurethane materials for therapy of chronic wounds". Biomaterials. 26 (30): 6024–33. doi:10.1016/j.biomaterials.2005.03.015. PMID 15885771.
- ↑ Gist S, Tio-Matos I, Falzgraf S, Cameron S, Beebe M (June 2009). "Wound care in the geriatric client". Clinical Interventions in Aging. 4: 269–87. doi: 10.2147/CIA.S4726 . PMC 2697592 . PMID 19554098.
- 1 2 3 4 5 6 7 Edwards JV, Howley P, Cohen IK (October 2004). "In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings". International Journal of Pharmaceutics. 284 (1–2): 1–12. doi:10.1016/j.ijpharm.2004.06.003. PMID 15454291.[ permanent dead link ]
- 1 2 3 4 5 6 7 Schönfelder U, Abel M, Wiegand C, Klemm D, Elsner P, Hipler UC (November 2005). "Influence of selected wound dressings on PMN elastase in chronic wound fluid and their antioxidative potential in vitro". Biomaterials. 26 (33): 6664–73. doi:10.1016/j.biomaterials.2005.04.030. PMID 15978664.
- 1 2 Augustin M, Maier K (2003). "Psychosomatic aspects of chronic wounds". Dermatology and Psychosomatics/Dermatologie und Psychosomatik. 4 (1): 5–13. doi:10.1159/000070529. S2CID 72066898.
- 1 2 3 4 5 6 7 8 Moreo K (2005). "Understanding and overcoming the challenges of effective case management for patients with chronic wounds". The Case Manager. 16 (2): 62–3, 67. doi:10.1016/j.casemgr.2005.01.014. PMID 15818347.
- ↑ Krasner D (May 1998). "Painful venous ulcers: themes and stories about living with the pain and suffering". Journal of Wound, Ostomy, and Continence Nursing. 25 (3): 158–68. doi:10.1097/00152192-199805000-00008. PMID 9678007.
- ↑ Hofman D, Ryan TJ, Arnold F, Cherry GW, Lindholm C, Bjellerup M, Glynn C (May 1997). "Pain in venous leg ulcers". Journal of Wound Care. 6 (5): 222–4. doi:10.12968/jowc.1997.6.5.222. PMID 9256727.
- ↑ Walshe C (December 1995). "Living with a venous leg ulcer: a descriptive study of patients' experiences". Journal of Advanced Nursing. 22 (6): 1092–100. doi:10.1111/j.1365-2648.1995.tb03110.x. PMID 8675863.
- ↑ Bardhan, Ajoy; Bruckner-Tuderman, Leena; Chapple, Iain L. C.; Fine, Jo-David; Harper, Natasha; Has, Cristina; Magin, Thomas M.; Marinkovich, M. Peter; Marshall, John F.; McGrath, John A.; Mellerio, Jemima E. (2020-09-24). "Epidermolysis bullosa". Nature Reviews Disease Primers. 6 (1): 78. doi:10.1038/s41572-020-0210-0. ISSN 2056-676X. PMID 32973163. S2CID 221861310.
- 1 2 Trent, JT. 2003. Wounds and malignancy. Archived 2016-01-13 at the Wayback Machine Advances in Skin & Wound Care. Accessed January 1, 2007.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 Mustoe T (May 2004). "Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy". American Journal of Surgery. 187 (5A): 65S–70S. doi:10.1016/S0002-9610(03)00306-4. PMID 15147994.
- ↑ Williams AM, Southern SJ (October 2005). "Conflicts in the treatment of chronic ulcers in drug addicts--case series and discussion". British Journal of Plastic Surgery. 58 (7): 997–9. doi: 10.1016/j.bjps.2005.04.024 . PMID 16040018.
- ↑ Vennemann B, Perdekamp MG, Weinmann W, Faller-Marquardt M, Pollak S, Brandis M (May 2006). "A case of Munchausen syndrome by proxy with subsequent suicide of the mother". Forensic Science International. 158 (2–3): 195–9. doi:10.1016/j.forsciint.2005.07.014. PMID 16169176.
- 1 2 Dowsett C, Gronemann MN, Harding K (2015). "Taking wound assessment beyond the edge". Wounds International. 6 (1). Archived from the original on 2018-05-04. Retrieved 2017-03-31.
- 1 2 3 4 5 6 7 8 Crovetti G, Martinelli G, Issi M, Barone M, Guizzardi M, Campanati B, et al. (April 2004). "Platelet gel for healing cutaneous chronic wounds". Transfusion and Apheresis Science. 30 (2): 145–51. doi:10.1016/j.transci.2004.01.004. PMID 15062754.
- 1 2 3 4 Alleva R, Nasole E, Di Donato F, Borghi B, Neuzil J, Tomasetti M (July 2005). "alpha-Lipoic acid supplementation inhibits oxidative damage, accelerating chronic wound healing in patients undergoing hyperbaric oxygen therapy". Biochemical and Biophysical Research Communications. 333 (2): 404–10. doi:10.1016/j.bbrc.2005.05.119. PMC 2136431 . PMID 15950945.
- ↑ Krishnaswamy VR, Manikandan M, Munirajan AK, Vijayaraghavan D, Korrapati PS (December 2014). "Expression and integrity of dermatopontin in chronic cutaneous wounds: a crucial factor in impaired wound healing". Cell and Tissue Research. 358 (3): 833–41. doi:10.1007/s00441-014-2000-z. PMID 25260909. S2CID 16355532.
- ↑ Lasagni L, Sagrinati C, Ronconi E, Angelotti ML, Parente E, Ballerini L, et al. (2010). "Novel strategies of regenerative medicine using chemical compounds". Current Medicinal Chemistry. 17 (34): 4134–49. doi:10.2174/092986710793348590. PMID 20939819.
- ↑ Mustoe T (May 2004). "Understanding chronic wounds: a unifying hypothesis on their pathogenesis and implications for therapy". American Journal of Surgery (review). 187 (5A): 65S–70S. doi:10.1016/S0002-9610(03)00306-4. PMID 15147994.
- ↑ Dhall S, Do D, Garcia M, Wijesinghe DS, Brandon A, Kim J, et al. (2014). "A novel model of chronic wounds: importance of redox imbalance and biofilm-forming bacteria for establishment of chronicity". PLOS ONE. 9 (10): e109848. Bibcode:2014PLoSO...9j9848D. doi: 10.1371/journal.pone.0109848 . PMC 4196950 . PMID 25313558.
- 1 2 3 Halcón L, Milkus K (November 2004). "Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial". American Journal of Infection Control. 32 (7): 402–8. doi:10.1016/j.ajic.2003.12.008. PMID 15525915.
- 1 2 Foy Y, Li J, Kirsner R, Eaglstein W (2004). "Analysis of fibroblast defects in extracellular matrix production in chronic wounds". Journal of the American Academy of Dermatology. 50 (3): P168. doi: 10.1016/j.jaad.2003.10.595 .
- 1 2 Kanda N, Watanabe S (April 2005). "Regulatory roles of sex hormones in cutaneous biology and immunology". Journal of Dermatological Science. 38 (1): 1–7. doi:10.1016/j.jdermsci.2004.10.011. PMID 15795118.
- 1 2 3 Lai JY, Borson ND, Strausbauch MA, Pittelkow MR (April 2004). "Mitosis increases levels of secretory leukocyte protease inhibitor in keratinocytes". Biochemical and Biophysical Research Communications. 316 (2): 407–10. doi:10.1016/j.bbrc.2004.02.065. PMID 15020232.
- 1 2 3 Reddy M, Gill SS, Wu W, Kalkar SR, Rochon PA (February 2012). "Does this patient have an infection of a chronic wound?". JAMA. 307 (6): 605–11. doi:10.1001/jama.2012.98. PMID 22318282.
- 1 2 3 Velander PE, Theopold C, Gheerardyn R, Bleiziffer O, Yao F, Eriksson E (2004). "Autologous cultured keratinocytes suspensions accelerate re-epithelialization in the diabetic pig". Journal of the American College of Surgeons. 199 (3): 58. doi:10.1016/j.jamcollsurg.2004.05.119.
- 1 2 3 Supp DM, Boyce ST (2005). "Engineered skin substitutes: practices and potentials". Clinics in Dermatology. 23 (4): 403–12. doi:10.1016/j.clindermatol.2004.07.023. PMID 16023936.
- 1 2 3 Thomas DR, Diebold MR, Eggemeyer LM (2005). "A controlled, randomized, comparative study of a radiant heat bandage on the healing of stage 3-4 pressure ulcers: a pilot study". Journal of the American Medical Directors Association. 6 (1): 46–9. doi:10.1016/j.jamda.2004.12.007. PMID 15871870.
- 1 2 Pressure ulcers: Surgical treatment and principles at eMedicine
- ↑ Ramasubbu DA, Smith V, Hayden F, Cronin P, et al. (Cochrane Wounds Group) (August 2017). "Systemic antibiotics for treating malignant wounds". The Cochrane Database of Systematic Reviews. 2017 (8): CD011609. doi:10.1002/14651858.CD011609.pub2. PMC 6483739 . PMID 28837757.
- ↑ Vermeulen, Hester; van Hattem, Jarne M; Storm-Versloot, Marja N; Ubbink, Dirk T; Westerbos, Stijn Joël (2007-01-24). Cochrane Wounds Group (ed.). "Topical silver for treating infected wounds". Cochrane Database of Systematic Reviews (1): CD005486. doi:10.1002/14651858.CD005486.pub2. PMID 17253557.
- ↑ "Anti-infective management of infected skin ulcers" (PDF). Infezioni in Medicina. 32 (2). 2024-06-01. doi:10.53854/liim-3202-3. PMC 11142418 . PMID 38827836.
- ↑ Jones V, Grey JE, Harding KG (April 2006). "Wound dressings". BMJ. 332 (7544): 777–80. doi:10.1136/bmj.332.7544.777. PMC 1420733 . PMID 16575081.
- ↑ Cutting K (May 2010). "Wound dressings: 21st century performance requirements". Journal of Wound Care. 19(Sup 1): 4–9. doi:10.12968/jowc.2010.19.Sup1.48258.
- 1 2 3 Brem H, Kirsner RS, Falanga V (July 2004). "Protocol for the successful treatment of venous ulcers". American Journal of Surgery. 188 (1A Suppl): 1–8. doi: 10.1016/S0002-9610(03)00284-8 . PMID 15223495.
- 1 2 3 Patel CV, Powell L, Wilson SE (2000). "Surgical wound infections". Current Treatment Options in Infectious Diseases. 2: 147–53. ISSN 1523-3820.
- ↑ Wolcott R, Fischenich RN (April 2014). "Ultimate Standardization of First-Line Wound Dressings to a Single Type". Today's Wound Clinic. 8 (3).
- ↑ Reyzelman AM, Vartivarian M (August 2015). "Evidence of Intensive Autolytic Debridement With a Self-Adaptive Wound Dressing". Wounds. 27 (8): 229–35. PMID 26284377.
- ↑ Newman GR, Walker M, Hobot JA, Bowler PG (March 2006). "Visualisation of bacterial sequestration and bactericidal activity within hydrating Hydrofiber wound dressings". Biomaterials. 27 (7): 1129–39. doi:10.1016/j.biomaterials.2005.07.046. PMID 16120458.
- ↑ Flanagan M, Vogensen H, and Haase L. 2006. Case series investigating the experience of pain in patients with chronic venous leg ulcers treated with a foam dressing releasing ibuprofen. World Wide Wounds. 2006
- ↑ Osterbrink J (2003). "Der Deutsche Schmerzstandard und seine Auswirkungen auf die Pflege". Die Schwester, der Pfleger. 42: 758–64.
- ↑ Kranke P, Bennett MH, Martyn-St James M, Schnabel A, Debus SE, Weibel S (June 2015). "Hyperbaric oxygen therapy for chronic wounds" (PDF). The Cochrane Database of Systematic Reviews. 2015 (6): CD004123. doi:10.1002/14651858.CD004123.pub4. PMC 7055586 . PMID 26106870.
- ↑ Greenhagen RM, Johnson AR, Peterson MC, Rogers LC, Bevilacqua NJ (2010). "Gastrocnemius recession as an alternative to tendoAchillis lengthening for relief of forefoot pressure in a patient with peripheral neuropathy: a case report and description of a technical modification". The Journal of Foot and Ankle Surgery. 49 (2): 159.e9–13. doi:10.1053/j.jfas.2009.07.002. PMID 20137982.
- ↑ Pop MA, Almquist BD (September 2017). "Biomaterials: A potential pathway to healing chronic wounds?". Experimental Dermatology. 26 (9): 760–763. doi:10.1111/exd.13290. PMC 5500184 . PMID 28094868.
- ↑ Postlethwaite AE, Kang AH (June 1976). "Collagen-and collagen peptide-induced chemotaxis of human blood monocytes". The Journal of Experimental Medicine. 143 (6): 1299–307. doi:10.1084/jem.143.6.1299. PMC 2190221 . PMID 1271012.