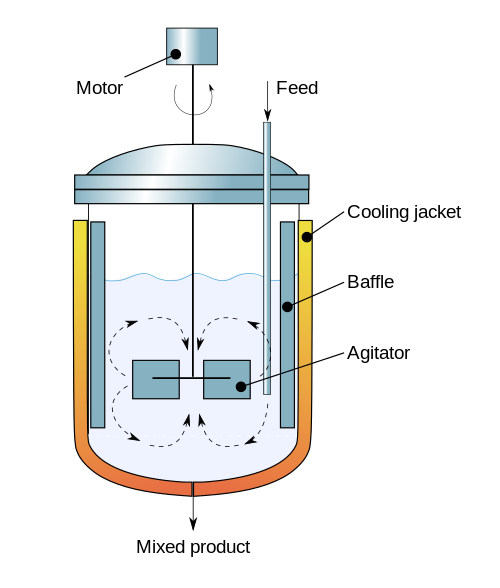
The continuous stirred-tank reactor (CSTR), also known as vat- or backmix reactor, mixed flow reactor (MFR), or a continuous-flow stirred-tank reactor (CFSTR), is a common model for a chemical reactor in chemical engineering and environmental engineering. A CSTR often refers to a model used to estimate the key unit operation variables when using a continuous agitated-tank reactor to reach a specified output. The mathematical model works for all fluids: liquids, gases, and slurries.
Contents
- Ideal CSTR
- Modeling
- Residence time distribution
- Non-ideal CSTR
- Modeling non-ideal flow
- Cascades of CSTRs
- Minimizing volume
- Ideal cascade of CSTRs
- Non-ideal cascade of CSTRs
- Cost
- Selectivity of parallel reactions
- Applications
- Environmental engineering
- Chemical engineering
- See also
- Notes
- References
The behavior of a CSTR is often approximated or modeled by that of an ideal CSTR, which assumes perfect mixing. In a perfectly mixed reactor, reagent is instantaneously and uniformly mixed throughout the reactor upon entry. Consequently, the output composition is identical to composition of the material inside the reactor, which is a function of residence time and reaction rate. The CSTR is the ideal limit of complete mixing in reactor design, which is the complete opposite of a plug flow reactor (PFR). In practice, no reactors behave ideally but instead fall somewhere in between the mixing limits of an ideal CSTR and PFR.