
Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy.

Turmeric or Curcuma longa, is a flowering plant in the ginger family Zingiberaceae. It is a perennial, rhizomatous, herbaceous plant native to the Indian subcontinent and Southeast Asia that requires temperatures between 20 and 30 °C and high annual rainfall to thrive. Plants are gathered each year for their rhizomes, some for propagation in the following season and some for consumption.

An acetate is a salt formed by the combination of acetic acid with a base. "Acetate" also describes the conjugate base or ion typically found in aqueous solution and written with the chemical formula C
2H
3O−
2. The neutral molecules formed by the combination of the acetate ion and a positive ion are also commonly called "acetates". The simplest of these is hydrogen acetate with corresponding salts, esters, and the polyatomic anion CH
3CO−
2, or CH
3COO−
.

A tincture is typically an extract of plant or animal material dissolved in ethanol. Solvent concentrations of 25–60% are common, but may run as high as 90%. In chemistry, a tincture is a solution that has ethanol as its solvent. In herbal medicine, alcoholic tinctures are made with various ethanol concentrations, which should be at least 20% alcohol for preservation purposes.

Lovastatin, sold under the brand name Mevacor among others, is a statin medication, to treat high blood cholesterol and reduce the risk of cardiovascular disease. Its use is recommended together with lifestyle changes. It is taken by mouth.

Alpinia nutans, the shellflower, or dwarf cardamom, is a Southeast Asian plant of the ginger family (Zingiberaceae), and is a medicinal plant used to control hypertension, as diuretic, antifungal, and antiulcer. In Japan it is used as food preservative.
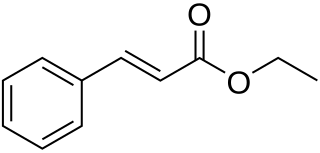
Ethyl cinnamate is the ester of cinnamic acid and ethanol. It is present in the essential oil of cinnamon. Pure ethyl cinnamate has a "fruity and balsamic odor, reminiscent of cinnamon with an amber note".

Gingerol ([6]-gingerol) is a phenolic phytochemical compound found in fresh ginger that activates spice receptors on the tongue. It is normally found as a pungent yellow oil in the ginger rhizome, but can also form a low-melting crystalline solid. This chemical compound is found in all members of the Zingiberaceae family and is high in concentrations in the grains of paradise as well as an African Ginger species.
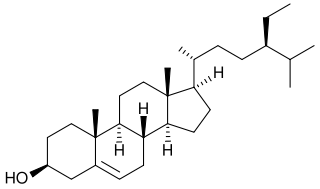
β-sitosterol (beta-sitosterol) is one of several phytosterols with chemical structures similar to that of cholesterol. It is a white, waxy powder with a characteristic odor, and is one of the components of the food additive E499. Phytosterols are hydrophobic and soluble in alcohols.

Trenbolone acetate, sold under brand names such as Finajet and Finaplix among others, is an androgen and anabolic steroid (AAS) medication which is used in veterinary medicine, specifically to increase the profitability of livestock by promoting muscle growth in cattle. It is given by injection into muscle.

Kaempferia galanga, commonly known as kencur, aromatic ginger, sand ginger, cutcherry, is a monocotyledonous plant in the ginger family, and one of four plants called galangal. It is found primarily in open areas in Indonesia, southern China, Taiwan, Cambodia, and India, but is also widely cultivated throughout Southeast Asia.

Coumestrol is a natural organic compound in the class of phytochemicals known as coumestans. Coumestrol was first identified as a compound with estrogenic properties by E. M. Bickoff in ladino clover and alfalfa in 1957. It has garnered research interest because of its estrogenic activity and prevalence in some foods, including soybeans, brussels sprouts, spinach and a variety of legumes. The highest concentrations of coumestrol are found in clover, Kala Chana, and Alfalfa sprouts.

The phenolic content in wine refers to the phenolic compounds—natural phenol and polyphenols—in wine, which include a large group of several hundred chemical compounds that affect the taste, color and mouthfeel of wine. These compounds include phenolic acids, stilbenoids, flavonols, dihydroflavonols, anthocyanins, flavanol monomers (catechins) and flavanol polymers (proanthocyanidins). This large group of natural phenols can be broadly separated into two categories, flavonoids and non-flavonoids. Flavonoids include the anthocyanins and tannins which contribute to the color and mouthfeel of the wine. The non-flavonoids include the stilbenoids such as resveratrol and phenolic acids such as benzoic, caffeic and cinnamic acids.

Genistin is an isoflavone found in a number of dietary plants like soy and kudzu. It was first isolated in 1931 from the 90% methanol extract of a soybean meal, when it was found that hydrolysis with hydrochloric acid produced 1 mole each of genistein and glucose. Chemically it is the 7-O-beta-D-glucoside form of genistein and is the predominant form of the isoflavone naturally occurring in plants. In fact, studies in the 1970s revealed that 99% of the isoflavonoid compounds in soy are present as their glucosides. The glucosides are converted by digestive enzymes in the digestive system to exert their biological effects. Genistin is also converted to a more familiar genistein, thus, the biological activities including antiatherosclerotic, estrogenic and anticancer effects are analogous.

Ptaquiloside is a norsesquiterpene glucoside produced by bracken ferns during metabolism. It is identified to be the main carcinogen of the ferns and to be responsible for their biological effects, such as haemorrhagic disease and bright blindness in livestock and oesophageal, gastric cancer in humans. Ptaquiloside has unstable chemical structure and acts as a DNA alkylating agent under physiological conditions. It was first isolated and characterized by Yamada and co-workers in 1983.
Alpinia nigra is a medium-sized herb belonging to the ginger family. The rhizome is well known in many Asian cultures as a medicinal and culinary item. In many Asian tribal communities it is a part of the diet along with rice.

Chrysophanol, also known as chrysophanic acid, is a fungal isolate and a natural anthraquinone. It is a C-3 methyl substituted chrysazin of the trihydroxyanthraquinone family.
Ethyl octanoate, also known as ethyl caprylate, is a fatty acid ester formed from caprylic acid and ethanol. A colorless liquid at room temperature, it has the semi-developed formula of CH3(CH2)6COOCH2CH3, and is used in food industries as a flavoring and in the perfume industry as a scent additive. It is present in many fruits and alcoholic beverages, and has a strong odor of fruit and flowers. It is used in the creation of synthetic fruity scents.
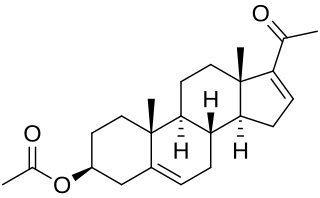
16-Dehydropregnenolone acetate (16-DPA) is a chemical compound used as an intermediate or synthon in the production of many semisynthetic steroids. As 7-ACA is for cephalosporins and 6-APA is for penicillins, 16-DPA is for steroids. While it is not easy to synthesize, it is a convenient intermediate which can be made from other more available materials, and which can then be modified to produce the desired target compound.

2-Methoxyestriol (2-MeO-E3) is an endogenous estrogen metabolite. It is specifically a metabolite of estriol and 2-hydroxyestriol. It has negligible affinity for the estrogen receptors and no estrogenic activity. However, 2-methoxyestriol does have some non-estrogen receptor-mediated cholesterol-lowering effects.

















