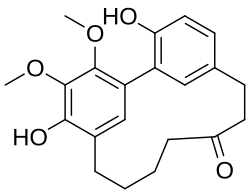
The diarylheptanoids (also known as diphenylheptanoids) are a class of plant secondary metabolites. Diarylheptanoids consist of two aromatic rings (aryl groups) joined by a seven carbons chain (heptane) and having various substituents. [1] They can be classified into linear (curcuminoids) and cyclic diarylheptanoids. The best known member is curcumin, which is isolated from turmeric (Curcuma longa) and is known as food coloring E100. Some other Curcuma species, such as Curcuma comosa also produce diarylheptanoids.
Contents
They have been reported from plants in 10 different families, e.g. Betulaceae and Zingiberaceae .
A diarylheptanoid is an intermediate in the biosynthesis of phenylphenalenones in Anigozanthos preissii [2] or Wachendorfia thyrsiflora (Haemodoraceae). [3]