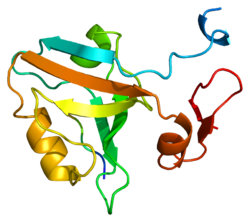
PSD-95 (postsynaptic density protein 95) also known as SAP-90 (synapse-associated protein 90) is a protein that in humans is encoded by the DLG4 (discs large homolog 4) gene. [5] [6] [7]
Contents
PSD-95 is a member of the membrane-associated guanylate kinase (MAGUK) family. With PSD-93 it is recruited into the same NMDA receptor and potassium channel clusters. These two MAGUK proteins may interact at postsynaptic sites to form a multimeric scaffold for the clustering of receptors, ion channels, and associated signaling proteins. [5] PSD-95 is the best studied member of the MAGUK-family of PDZ domain-containing proteins. Like all MAGUK-family proteins, its basic structure includes three PDZ domains, an SH3 domain, and a guanylate kinase-like domain (GK) connected by disordered linker regions. It is almost exclusively located in the post synaptic density of neurons, [8] and is involved in anchoring synaptic proteins. Its direct and indirect binding partners include neuroligin, NMDA receptors, AMPA receptors, and potassium channels. [9] It plays an important role in synaptic plasticity and the stabilization of synaptic changes during long-term potentiation. [10]