
The molars or molar teeth are large, flat teeth at the back of the mouth. They are more developed in mammals. They are used primarily to grind food during chewing. The name molar derives from Latin, molaris dens, meaning "millstone tooth", from mola, millstone and dens, tooth. Molars show a great deal of diversity in size and shape across mammal groups. The third molar of humans is sometimes vestigial.
Ferugliotherium is a genus of fossil mammals in the family Ferugliotheriidae from the Campanian and/or Maastrichtian period of Argentina. It contains a single species, Ferugliotherium windhauseni, which was first described in 1986. Although originally interpreted on the basis of a single brachydont (low-crowned) molar as a member of Multituberculata, an extinct group of small, rodent-like mammals, it was recognized as related to the hypsodont (high-crowned) Sudamericidae following the discovery of additional material in the early 1990s. After a jaw of the sudamericid Sudamerica was described in 1999, these animals were no longer considered to be multituberculates and a few fossils that were previously considered to be Ferugliotherium were assigned to unspecified multituberculates instead. Since 2005, a relationship between gondwanatheres and multituberculates has again received support. A closely related animal, Trapalcotherium, was described in 2009 on the basis of a single tooth.

Colugos are arboreal gliding mammals that are native to Southeast Asia. Their closest evolutionary relatives are primates. There are just two living species of colugos: the Sunda flying lemur and the Philippine flying lemur. These two species make up the entire family Cynocephalidae and order Dermoptera.
Tingamainus is a species of early placenta bearing mammal.

Ambondro mahabo is a mammal from the Middle Jurassic (Bathonian) Isalo III Formation of Madagascar. The only described species of the genus Ambondro, it is known from a fragmentary lower jaw with three teeth, interpreted as the last premolar and the first two molars. The premolar consists of a central cusp with one or two smaller cusps and a cingulum (shelf) on the inner, or lingual, side of the tooth. The molars also have such a lingual cingulum. They consist of two groups of cusps: a trigonid of three cusps at the front and a talonid with a main cusp, a smaller cusp, and a crest at the back. Features of the talonid suggest that Ambondro had tribosphenic molars, the basic arrangement of molar features also present in marsupial and placental mammals. It is the oldest known mammal with putatively tribosphenic teeth; at the time of its discovery it antedated the second oldest example by about 25 million years.
Many different terms have been proposed for features of the tooth crown in mammals.
UA 8699 is a fossil mammalian tooth from the Cretaceous of Madagascar. A broken lower molar about 3.5 mm (0.14 in) long, it is from the Maastrichtian of the Maevarano Formation in northwestern Madagascar. Details of its crown morphology indicate that it is a boreosphenidan, a member of the group that includes living marsupials and placental mammals. David W. Krause, who first described the tooth in 2001, interpreted it as a marsupial on the basis of five shared characters, but in 2003 Averianov and others noted that all those are shared by zhelestid placentals and favored a close relationship between UA 8699 and the Spanish zhelestid Lainodon. Krause used the tooth as evidence that marsupials were present on the southern continents (Gondwana) as early as the late Cretaceous and Averianov and colleagues proposed that the tooth represented another example of faunal exchange between Africa and Europe at the time.

Brachytarsomys mahajambaensis is an extinct rodent from northwestern Madagascar. It is known from nine isolated molars found in several sites during fieldwork that started in 2001. First described in 2010, it is placed in the genus Brachytarsomys together with two larger living species, which may differ in some details of molar morphology. The presence of B. mahajambaensis, a rare element in the local rodent fauna, suggests that the region was previously more humid.

Nesomys narindaensis is an extinct rodent that lived in northwestern Madagascar. It is known from subfossil skull bones and isolated molars found in several sites during field work that started in 2001. First described in 2010, it is placed in the genus Nesomys together with three smaller living species, which may differ in some details of molar morphology. The presence of N. narindaensis, a rare element in the local rodent fauna, suggests that the region was previously more humid.

Hipposideros besaoka is an extinct bat from Madagascar in the genus Hipposideros. It is known from numerous jaws and teeth, which were collected in a cave at Anjohibe in 1996 and described as a new species in 2007. The site where H. besaoka was found is at most 10,000 years old; other parts of the cave have yielded H. commersoni, a living species of Hipposideros from Madagascar, and some material that is distinct from both species. H. besaoka was larger than H. commersoni, making it the largest insectivorous bat of Madagascar, and had broader molars and a more robust lower jaw. As usual in Hipposideros, the second upper premolar is small and displaced from the toothrow, and the second lower premolar is large.

Triaenops goodmani is an extinct bat from Madagascar in the genus Triaenops. It is known from three lower jaws collected in a cave at Anjohibe in 1996, and described as a new species in 2007. The material is at most 10,000 years old. A bat humerus from the same site could not be identified as either T. goodmani or the living T. menamena. T. goodmani is identifiable as a member of Triaenops or the related genus Paratriaenops by a number of features of the teeth, such as the single-cusped, canine-like fourth premolar and the presence of a gap between the entoconid and hypoconulid cusps on the first two molars. T. goodmani is larger than the living species of Triaenops and Paratriaenops on Madagascar, and on the first molar the protoconid cusp is only slightly higher than the hypoconid, not much higher as in the other species.
Lagrivea is a fossil genus of squirrel from the Middle Miocene of France. The single species, L. vireti, is known from three mandibles and two isolated teeth. All come from the fissure filling of La Grive L5, part of the La Grive-Saint-Alban complex in Saint-Alban-de-Roche, southeastern France. Lagrivea was a large tree squirrel with flat lower incisors and a large, triangular fourth lower premolar (p4). Each of the four cheekteeth bears a deep basin in the middle of the crown. The m3 is about rectangular in shape, but rounded at the back. Although m1 and m2 have two roots, m3 has three.
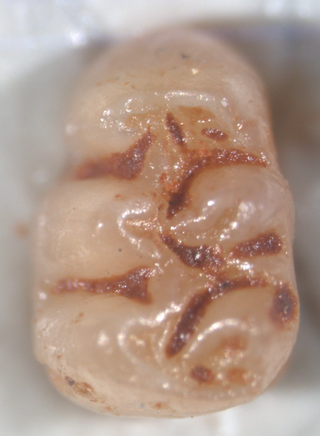
Agathaeromys is an extinct genus of oryzomyine rodents from the Pleistocene of Bonaire, Netherlands Antilles. Two species are known, which differ in size and some details of tooth morphology. The larger A. donovani, the type species, is known from hundreds of teeth that are probably 900,000 to 540,000 years old, found in four localities. A. praeuniversitatis, the smaller species, is known from 35 teeth found in a single fossil site, which is probably 540,000 to 230,000 years old.
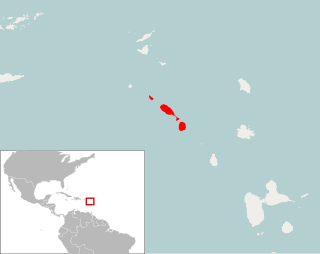
Pennatomys nivalis is an extinct oryzomyine rodent from the islands of Sint Eustatius, Saint Kitts, and Nevis in the Lesser Antilles. The only species in the genus Pennatomys, it is known from skeletal remains found in Amerindian archeological sites on all three islands, with dates ranging from 790–520 BCE to 900–1200 CE. No live specimens are known, but there are several historical records of rodents from Saint Kitts and Nevis that could conceivably refer to Pennatomys. The animal apparently belongs to a group within the tribe Oryzomyini that includes many other island-dwelling species.
Donodontidae is an extinct family of cladotherian mammals known from the Late Jurassic and Early Cretaceous of North Africa. When originally named in 1991, Donodontidae was a monotypic family containing a single species: Donodon perscriptoris. In 2022, four more species were designated and placed within the family: Donodon minor, Stylodens amerrukensis, Anoualestes incidens, and Amazighodon orbis. All five species are endemic to the Ksar Metlili Formation of Morocco, which is dated to the Tithonian and Berriasian. Donodontid fossils are restricted to postcanine teeth and associated jaw fragments.
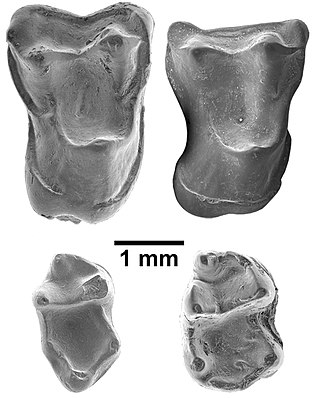
Afrasia djijidae is a fossil primate that lived in Myanmar approximately 37 million years ago, during the late middle Eocene. The only species in the genus Afrasia, it was a small primate, estimated to weigh around 100 grams (3.5 oz). Despite the significant geographic distance between them, Afrasia is thought to be closely related to Afrotarsius, an enigmatic fossil found in Libya and Egypt that dates to 38–39 million years ago. If this relationship is correct, it suggests that early simians dispersed from Asia to Africa during the middle Eocene and would add further support to the hypothesis that the first simians evolved in Asia, not Africa. Neither Afrasia nor Afrotarsius, which together form the family Afrotarsiidae, is considered ancestral to living simians, but they are part of a side branch or stem group known as eosimiiforms. Because they did not give rise to the stem simians that are known from the same deposits in Africa, early Asian simians are thought to have dispersed from Asia to Africa more than once prior to the late middle Eocene. Such dispersals from Asia to Africa also were seen around the same time in other mammalian groups, including hystricognathous rodents and anthracotheres.
Indraloris is a fossil primate from the Miocene of India and Pakistan in the family Sivaladapidae. Two species are now recognized: I. himalayensis from Haritalyangar, India and I. kamlialensis from the Pothohar Plateau, Pakistan. Other material from the Potwar Plateau may represent an additional, unnamed species. Body mass estimates range from about 2 kg (4.4 lb) for the smaller I. kamlialensis to over 4 kg (8.8 lb) for the larger I. himalayensis.
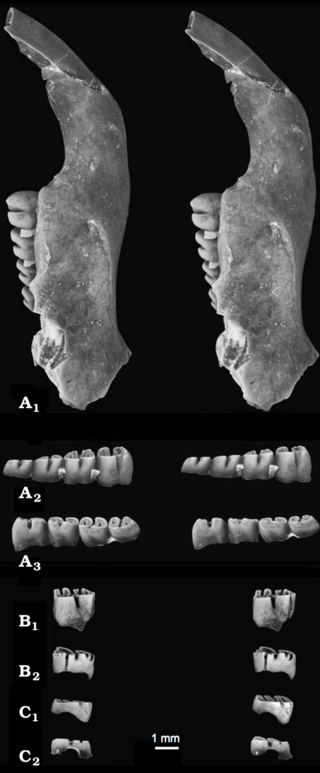
Apeomyoides savagei is a fossil rodent from the Miocene of the United States, the only species in the genus Apeomyoides. It is known from fragmentary jaws and isolated teeth from a site in the early Barstovian, around 15–16 million years ago, of Nevada. Together with other species from scattered localities in the United States, Japan, and Europe, Apeomyoides is classified in the subfamily Apeomyinae of the extinct rodent family Eomyidae. Apeomyines are a rare but widespread group that may have been adapted to a relatively dry habitat.
Sivaladapis is a genus of adapiform primate that lived in Asia during the middle Miocene.
Exiguodon is an extinct genus of hyainailourid hyaenodont mammal of the subfamily Hyainailourinae. Remains are known from early Miocene deposits in Kenya and Uganda, in East Africa.

















