
Galactosemia is a rare genetic metabolic disorder that affects an individual's ability to metabolize the sugar galactose properly. Galactosemia follows an autosomal recessive mode of inheritance that confers a deficiency in an enzyme responsible for adequate galactose degradation.

Hereditary coproporphyria (HCP) is a disorder of heme biosynthesis, classified as an acute hepatic porphyria. HCP is caused by a deficiency of the enzyme coproporphyrinogen oxidase, coded for by the CPOX gene, and is inherited in an autosomal dominant fashion, although homozygous individuals have been identified. Unlike acute intermittent porphyria, individuals with HCP can present with cutaneous findings similar to those found in porphyria cutanea tarda in addition to the acute attacks of abdominal pain, vomiting and neurological dysfunction characteristic of acute porphyrias. Like other porphyrias, attacks of HCP can be induced by certain drugs, environmental stressors or diet changes. Biochemical and molecular testing can be used to narrow down the diagnosis of a porphyria and identify the specific genetic defect. Overall, porphyrias are rare diseases. The combined incidence for all forms of the disease has been estimated at 1:20,000. The exact incidence of HCP is difficult to determine, due to its reduced penetrance.

Hyperammonemia, or high ammonia levels, is a metabolic disturbance characterised by an excess of ammonia in the blood. It is a dangerous condition that may lead to brain injury and death. It may be primary or secondary.

Medium-chain acyl-CoA dehydrogenase deficiency is a disorder of fatty acid oxidation that impairs the body's ability to break down medium-chain fatty acids into acetyl-CoA. The disorder is characterized by hypoglycemia and sudden death without timely intervention, most often brought on by periods of fasting or vomiting.

Hereditary fructose intolerance (HFI) is an inborn error of fructose metabolism caused by a deficiency of the enzyme aldolase B. Individuals affected with HFI are asymptomatic until they ingest fructose, sucrose, or sorbitol. If fructose is ingested, the enzymatic block at aldolase B causes an accumulation of fructose-1-phosphate which, over time, results in the death of liver cells. This accumulation has downstream effects on gluconeogenesis and regeneration of adenosine triphosphate (ATP). Symptoms of HFI include vomiting, convulsions, irritability, poor feeding as a baby, hypoglycemia, jaundice, hemorrhage, hepatomegaly, hyperuricemia and potentially kidney failure. While HFI is not clinically a devastating condition, there are reported deaths in infants and children as a result of the metabolic consequences of HFI. Death in HFI is always associated with problems in diagnosis.
Inborn errors of metabolism form a large class of genetic diseases involving congenital disorders of enzyme activities. The majority are due to defects of single genes that code for enzymes that facilitate conversion of various substances (substrates) into others (products). In most of the disorders, problems arise due to accumulation of substances which are toxic or interfere with normal function, or due to the effects of reduced ability to synthesize essential compounds. Inborn errors of metabolism are often referred to as congenital metabolic diseases or inherited metabolic disorders. Another term used to describe these disorders is "enzymopathies". This term was created following the study of biodynamic enzymology, a science based on the study of the enzymes and their products. Finally, inborn errors of metabolism were studied for the first time by British physician Archibald Garrod (1857–1936), in 1908. He is known for work that prefigured the "one gene–one enzyme" hypothesis, based on his studies on the nature and inheritance of alkaptonuria. His seminal text, Inborn Errors of Metabolism, was published in 1923.

Glycogen storage disease type I is an inherited disease that prevents the liver from properly breaking down stored glycogen, which is necessary to maintain adequate blood sugar levels. GSD I is divided into two main types, GSD Ia and GSD Ib, which differ in cause, presentation, and treatment. There are also possibly rarer subtypes, the translocases for inorganic phosphate or glucose ; however, a recent study suggests that the biochemical assays used to differentiate GSD Ic and GSD Id from GSD Ib are not reliable, and are therefore GSD Ib.

Maple syrup urine disease (MSUD) is a rare, inherited metabolic disorder that affects the body’s ability to metabolize amino acids due to a deficiency in the activity of the branched-chain alpha-ketoacid dehydrogenase (BCKAD) complex. It particularly affects the metabolism of amino acids- leucine, isoleucine, and valine. With MSUD, the body is not able to properly break down these amino acids, therefore leading to the amino acids to build up in urine and become toxic. The condition gets its name from the distinctive sweet odor of affected infants' urine and earwax due to the buildup of these amino acids.

Systemic primary carnitine deficiency (SPCD) is an inborn error of fatty acid transport caused by a defect in the transporter responsible for moving carnitine across the plasma membrane. Carnitine is an important amino acid for fatty acid metabolism. When carnitine cannot be transported into tissues, fatty acid oxidation is impaired, leading to a variety of symptoms such as chronic muscle weakness, cardiomyopathy, hypoglycemia and liver dysfunction. The specific transporter involved with SPCD is OCTN2, coded for by the SLC22A5 gene located on chromosome 5. SPCD is inherited in an autosomal recessive manner, with mutated alleles coming from both parents.
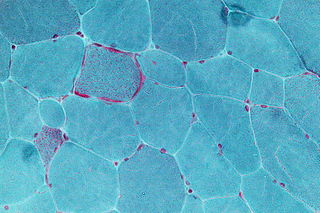
A metabolic disorder is a disorder that negatively alters the body's processing and distribution of macronutrients, such as proteins, fats, and carbohydrates. Metabolic disorders can happen when abnormal chemical reactions in the body alter the normal metabolic process. It can also be defined as inherited single gene anomaly, most of which are autosomal recessive.
Disaccharidases are glycoside hydrolases, enzymes that break down certain types of sugars called disaccharides into simpler sugars called monosaccharides. In the human body, disaccharidases are made mostly in an area of the small intestine's wall called the brush border, making them members of the group of "brush border enzymes".

Glycogen storage disease type 0 is a disease characterized by a deficiency in the glycogen synthase enzyme (GSY). Although glycogen synthase deficiency does not result in storage of extra glycogen in the liver, it is often classified as a glycogen storage disease because it is another defect of glycogen storage and can cause similar problems. There are two isoforms (types) of glycogen synthase enzyme; GSY1 in muscle and GSY2 in liver, each with a corresponding form of the disease. Mutations in the liver isoform (GSY2), causes fasting hypoglycemia, high blood ketones, increased free fatty acids and low levels of alanine and lactate. Conversely, feeding in these patients results in hyperglycemia and hyperlactatemia.
2,4 Dienoyl-CoA reductase deficiency is an inborn error of metabolism resulting in defective fatty acid oxidation caused by a deficiency of the enzyme 2,4 Dienoyl-CoA reductase. Lysine degradation is also affected in this disorder leading to hyperlysinemia. The disorder is inherited in an autosomal recessive manner, meaning an individual must inherit mutations in NADK2, located at 5p13.2 from both of their parents. NADK2 encodes the mitochondrial NAD kinase. A defect in this enzyme leads to deficient mitochondrial nicotinamide adenine dinucleotide phosphate levels. 2,4 Dienoyl-CoA reductase, but also lysine degradation are performed by NADP-dependent oxidoreductases explaining how NADK2 deficiency can lead to multiple enzyme defects.
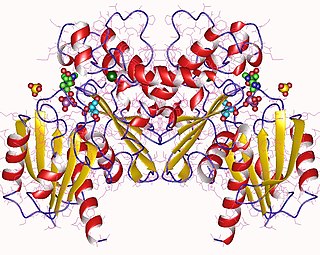
Fructokinase, also known as D-fructokinase or D-fructose (D-mannose) kinase, is an enzyme of the liver, intestine, and kidney cortex. Fructokinase is in a family of enzymes called transferases, meaning that this enzyme transfers functional groups; it is also considered a phosphotransferase since it specifically transfers a phosphate group. Fructokinase specifically catalyzes the transfer of a phosphate group from adenosine triphosphate to fructose as the initial step in its utilization. The main role of fructokinase is in carbohydrate metabolism, more specifically, sucrose and fructose metabolism. The reaction equation is as follows:
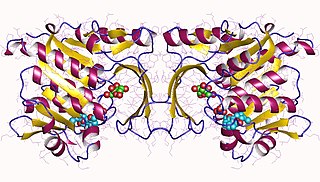
Hepatic fructokinase is an enzyme that catalyzes the phosphorylation of fructose to produce fructose-1-phosphate.

Galactose-1-phosphate uridylyltransferase deficiency(classic galactosemia) is the most common type of galactosemia, an inborn error of galactose metabolism, caused by a deficiency of the enzyme galactose-1-phosphate uridylyltransferase. It is an autosomal recessive metabolic disorder that can cause liver disease and death if untreated. Treatment of galactosemia is most successful if initiated early and includes dietary restriction of lactose intake. Because early intervention is key, galactosemia is included in newborn screening programs in many areas. On initial screening, which often involves measuring the concentration of galactose in blood, classic galactosemia may be indistinguishable from other inborn errors of galactose metabolism, including galactokinase deficiency and galactose epimerase deficiency. Further analysis of metabolites and enzyme activities are needed to identify the specific metabolic error.

Inborn errors of carbohydrate metabolism are inborn error of metabolism that affect the catabolism and anabolism of carbohydrates.
Fructolysis refers to the metabolism of fructose from dietary sources. Though the metabolism of glucose through glycolysis uses many of the same enzymes and intermediate structures as those in fructolysis, the two sugars have very different metabolic fates in human metabolism. Under one percent of ingested fructose is directly converted to plasma triglyceride. 29% - 54% of fructose is converted in liver to glucose, and about a quarter of fructose is converted to lactate. 15% - 18% is converted to glycogen. Glucose and lactate are then used normally as energy to fuel cells all over the body.
Congenital hemolytic anemia (CHA) is a diverse group of rare hereditary conditions marked by decreased life expectancy and premature removal of erythrocytes from blood flow. Defects in erythrocyte membrane proteins and red cell enzyme metabolism, as well as changes at the level of erythrocyte precursors, lead to impaired bone marrow erythropoiesis. CAH is distinguished by variable anemia, chronic extravascular hemolysis, decreased erythrocyte life span, splenomegaly, jaundice, biliary lithiasis, and iron overload. Immune-mediated mechanisms may play a role in the pathogenesis of these uncommon diseases, despite the paucity of data regarding the immune system's involvement in CHAs.

Ornithine aminotransferase deficiency is an inborn error of ornithine metabolism, caused by decreased activity of the enzyme ornithine aminotransferase. Biochemically, it can be detected by elevated levels of ornithine in the blood. Clinically, it presents initially with poor night vision, which slowly progresses to total blindness. It is believed to be inherited in an autosomal recessive manner. Approximately 200 known cases have been reported in the literature. The incidence is highest in Finland, estimated at 1:50,000.













