
Aspergillus terreus, also known as Aspergillus terrestris, is a fungus (mold) found worldwide in soil. Although thought to be strictly asexual until recently, A. terreus is now known to be capable of sexual reproduction. This saprotrophic fungus is prevalent in warmer climates such as tropical and subtropical regions. Aside from being located in soil, A. terreus has also been found in habitats such as decomposing vegetation and dust. A. terreus is commonly used in industry to produce important organic acids, such as itaconic acid and cis-aconitic acid, as well as enzymes, like xylanase. It was also the initial source for the drug mevinolin (lovastatin), a drug for lowering serum cholesterol.
Acremonium strictum is an environmentally widespread saprotroph species found in soil, plant debris, and rotting mushrooms. Isolates have been collected in North and Central America, Asia, Europe and Egypt. A. strictum is an agent of hyalohyphomycosis and has been identified as an increasingly frequent human pathogen in immunosuppressed individuals, causing localized, disseminated and invasive infections. Although extremely rare, A. strictum can infect immunocompetent individuals, as well as neonates. Due to the growing number of infections caused by A. strictum in the past few years, the need for new medical techniques in the identification of the fungus as well as for the treatment of human infections has risen considerably.

Acrophialophora fusispora is a poorly studied ascomycete fungus found in soil, air and various plants. A. fusispora is morphologically similar to the genera Paecilomyces and Masonia, but differ in the presence of pigmented conidiophores, verticillate phialides, and frequent sympodial proliferation. Moreover, A. fusispora is distinguished by its pigmented spindle-shaped conidia, covered with spiral bands. The fungus is naturally found in soils of tropical to temperate regions. The fungus has been identified as a plant and animal pathogen, and has recently been recognized as an emerging opportunistic human pathogen. A. fusispora infection in human is rare and has few documented clinical cases, but due to the rarity of the fungus and potential misidentification, the infections may be underdiagnosed. Clinical cases of A. fusispora include cases of keratitis, pulmonary colonization and infection, and cerebral infections. The fungus also has two documented cases of infection in dogs.
Nigrospora sphaerica is an airborne filamentous fungus in the phylum Ascomycota. It is found in soil, air, and plants as a leaf pathogen. It can occur as an endophyte where it produces antiviral and antifungal secondary metabolites. Sporulation of N. sphaerica causes its initial white coloured colonies to rapidly turn black. N. sphaerica is often confused with the closely related species N. oryzae due to their morphological similarities.

Exophiala is a genus of anamorphic fungi in the family Herpotrichiellaceae. The widespread genus contains 28 species. The genus was formally described by J. W. Carmichael in 1966.
Exophiala jeanselmei is a saprotrophic fungus in the family Herpotrichiellaceae. Four varieties have been discovered: Exophiala jeanselmei var. heteromorpha, E. jeanselmei var. lecanii-corni, E. jeanselmei var. jeanselmei, and E. jeanselmei var. castellanii. Other species in the genus Exophiala such as E. dermatitidis and E. spinifera have been reported to have similar annellidic conidiogenesis and may therefore be difficult to differentiate.
Trichophyton concentricum is an anthropophilic dermatophyte believed to be an etiological agent of a type of skin mycosis in humans, evidenced by scaly cutaneous patches on the body known as tinea imbricata. This fungus has been found mainly in the Pacific Islands and South America.

Exophiala dermatitidis is a thermophilic black yeast, and a member of the Herpotrichiellaceae. While the species is only found at low abundance in nature, metabolically active strains are commonly isolated in saunas, steam baths, and dish washers. Exophiala dermatitidis only rarely causes infection in humans, however cases have been reported around the world. In East Asia, the species has caused lethal brain infections in young and otherwise healthy individuals. The fungus has been known to cause cutaneous and subcutaneous phaeohyphomycosis, and as a lung colonist in people with cystic fibrosis in Europe. In 2002, an outbreak of systemic E. dermatitidis infection occurred in women who had received contaminated steroid injections at North Carolina hospitals.
Exophiala hongkongensis is an ascomycete fungus species. Described as new to science in 2013, it was discovered on the toenail clipping of a patient with the fungal disease onychomycosis.
Dark septate endophytes (DSE) are a group of endophytic fungi characterized by their morphology of melanized, septate, hyphae. This group is likely paraphyletic, and contain conidial as well as sterile fungi that colonize roots intracellularly or intercellularly. Very little is known about the number of fungal taxa within this group, but all are in the Ascomycota. They are found in over 600 plant species and across 114 families of angiosperms and gymnosperms and co-occur with other types of mycorrhizal fungi. They have a wide global distribution and can be more abundant in stressed environments. Much of their taxonomy, physiology, and ecology are unknown.
Emmonsia parva is a filamentous, saprotrophic fungus and one of three species within the genus Emmonsia. The fungus is most known for its causal association with the lung disease, adiaspiromycosis which occurs most commonly in small mammals but is also seen in humans. The disease was first described from rodents in Arizona, and the first human case was reported in France in 1964. Since then, the disease has been reported from Honduras, Brazil, the Czech Republic, Russia, the United States of America and Guatemala. Infections in general are quite rare, especially in humans.

Penicillium digitatum is a mesophilic fungus found in the soil of citrus-producing areas. It is a major source of post-harvest decay in fruits and is responsible for the widespread post-harvest disease in Citrus fruit known as green rot or green mould. In nature, this necrotrophic wound pathogen grows in filaments and reproduces asexually through the production of conidiophores and conidia. However, P. digitatum can also be cultivated in the laboratory setting. Alongside its pathogenic life cycle, P. digitatum is also involved in other human, animal and plant interactions and is currently being used in the production of immunologically based mycological detection assays for the food industry.

Trichothecium roseum is a fungus in the division Ascomycota first reported in 1809. It is characterized by its flat and granular colonies which are initially white and develop to be light pink in color. This fungus reproduces asexually through the formation of conidia with no known sexual state. Trichothecium roseum is distinctive from other species of the genus Trichothecium in its characteristic zigzag patterned chained conidia. It is found in various countries worldwide and can grow in a variety of habitats ranging from leaf litter to fruit crops. Trichothecium roseum produces a wide variety of secondary metabolites including mycotoxins, such as roseotoxins and trichothecenes, which can infect and spoil a variety of fruit crops. It can act as both a secondary and opportunistic pathogen by causing pink rot on various fruits and vegetables and thus has an economical impact on the farming industry. Secondary metabolites of T. roseum, specifically Trichothecinol A, are being investigated as potential anti-metastatic drugs. Several agents including harpin, silicon oxide, and sodium silicate are potential inhibitors of T. roseum growth on fruit crops. Trichothecium roseum is mainly a plant pathogen and has yet to show a significant impact on human health.
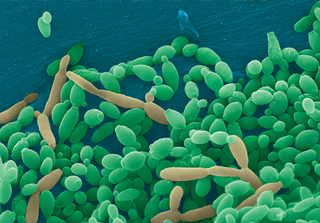
Candida tropicalis is a species of yeast in the genus Candida. It is a common pathogen in neutropenic hosts, in whom it may spread through the bloodstream to peripheral organs. For invasive disease, treatments include amphotericin B, echinocandins, or extended-spectrum triazole antifungals.

Phialophora verrucosa is a pathogenic, dematiaceous fungus that is a common cause of chromoblastomycosis. It has also been reported to cause subcutaneous phaeohyphomycosis and mycetoma in very rare cases. In the natural environment, it can be found in rotting wood, soil, wasp nests, and plant debris. P. verrucosa is sometimes referred to as Phialophora americana, a closely related environmental species which, along with P. verrucosa, is also categorized in the P. carrionii clade.
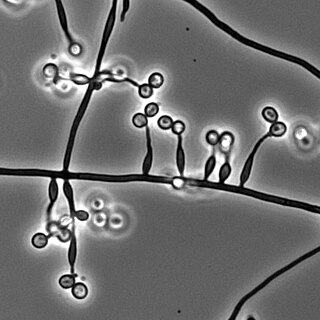
Metarhizium granulomatis is a fungus in the family Clavicipitaceae associated with systemic mycosis in veiled chameleons. The genus Metarhizium is known to infect arthropods, and collectively are referred to green-spored asexual pathogenic fungi. This species grows near the roots of plants and has been reported as an agent of disease in captive veiled chameleons. The etymology of the species epithet, "granulomatis" refers to the ability of the fungus to cause granulomatous disease in susceptible reptiles.
Sarocladium kiliense is a saprobic fungus that is occasionally encountered as a opportunistic pathogen of humans, particularly immunocompromised and individuals. The fungus is frequently found in soil and has been linked with skin and systemic infections. This species is also known to cause disease in the green alga, Cladophora glomerata as well as various fruit and vegetable crops grown in warmer climates.
Myriodontium keratinophilum is a fungus widespread in nature, most abundantly found in keratin-rich environments such as feathers, nails and hair. Despite its ability to colonize keratinous surfaces of human body, the species has been known to be non-pathogenic in man and is phylogentically distant to other human pathogenic species, such as anthropophilic dermatophytes. However, its occasional isolation from clinical specimens along with its keratinolytic properties suggest the possibility it may contribute to disease.
Cladophialophora arxii is a black yeast shaped dematiaceous fungus that is able to cause serious phaeohyphomycotic infections. C. arxii was first discovered in 1995 in Germany from a 22-year-old female patient suffering multiple granulomatous tracheal tumours. It is a clinical strain that is typically found in humans and is also capable of acting as an opportunistic fungus of other vertebrates Human cases caused by C. arxii have been reported from all parts of the world such as Germany and Australia.
Curvularia geniculata is a fast-growing anamorphic fungus in the division Ascomycota, most commonly found in soil, especially in areas of warmer climates. The fungus is a pathogen, mainly causing plant and animal infections, and rarely causing human infections. C. geniculata is characterized by its curved conidia, which has a dark brown centre and pale tapered tips, and produces anti-fungal compounds called Curvularides A-E.








