
Frost is a thin layer of ice on a solid surface, which forms from water vapor that deposits onto a freezing surface. Frost forms when the air contains more water vapor than it can normally hold at a specific temperature. The process is similar to the formation of dew, except it occurs below the freezing point of water typically without crossing through a liquid state.

Fog is a visible aerosol consisting of tiny water droplets or ice crystals suspended in the air at or near the Earth's surface. Fog can be considered a type of low-lying cloud usually resembling stratus, and is heavily influenced by nearby bodies of water, topography, and wind conditions. In turn, fog affects many human activities, such as shipping, travel, and warfare.
Diamond dust is a ground-level cloud composed of tiny ice crystals. This meteorological phenomenon is also referred to simply as ice crystals and is reported in the METAR code as IC. Diamond dust generally forms under otherwise clear or nearly clear skies, so it is sometimes referred to as clear-sky precipitation. Diamond dust is most commonly observed in Antarctica and the Arctic, but can occur anywhere with a temperature well below freezing. In the polar regions of Earth, diamond dust may persist for several days without interruption.

An ice storm, also known as a glaze event or a silver storm, is a type of winter storm characterized by freezing rain. The U.S. National Weather Service defines an ice storm as a storm which results in the accumulation of at least 0.25-inch (6.4 mm) of ice on exposed surfaces. They are generally not violent storms but instead are commonly perceived as gentle rains occurring at temperatures just below freezing.
Freezing rain is rain maintained at temperatures below freezing by the ambient air mass that causes freezing on contact with surfaces. Unlike a mixture of rain and snow or ice pellets, freezing rain is made entirely of liquid droplets. The raindrops become supercooled while passing through a sub-freezing layer of air hundreds of meters above the ground, and then freeze upon impact with any surface they encounter, including the ground, trees, electrical wires, aircraft, and automobiles. The resulting ice, called glaze ice, can accumulate to a thickness of several centimeters and cover all exposed surfaces. The METAR code for freezing rain is FZRA.

Ice crystals are solid ice in symmetrical shapes including hexagonal columns, hexagonal plates, and dendritic crystals. Ice crystals are responsible for various atmospheric optic displays and cloud formations.

Supercooling, also known as undercooling, is the process of lowering the temperature of a liquid below its freezing point without it becoming a solid. As per the established international definition, supercooling means ‘cooling a substance below the normal freezing point without solidification’ While it can be achieved by different physical means, the postponed solidification is most often due to the absence of seed crystals or nuclei around which a crystal structure can form. The supercooling of water can be achieved without any special techniques other than chemical demineralization, down to −48.3 °C (−54.9 °F). Supercooled water can occur naturally, for example in the atmosphere, animals or plants.

In meteorology, precipitation is any product of the condensation of atmospheric water vapor that falls from clouds due to gravitational pull. The main forms of precipitation include drizzle, rain, sleet, snow, ice pellets, graupel and hail. Precipitation occurs when a portion of the atmosphere becomes saturated with water vapor, so that the water condenses and "precipitates" or falls. Thus, fog and mist are not precipitation; their water vapor does not condense sufficiently to precipitate, so fog and mist do not fall. Two processes, possibly acting together, can lead to air becoming saturated with water vapor: cooling the air or adding water vapor to the air. Precipitation forms as smaller droplets coalesce via collision with other rain drops or ice crystals within a cloud. Short, intense periods of rain in scattered locations are called showers.
In physics and chemistry, flash freezing is the process whereby objects are rapidly frozen. This is done by subjecting them to cryogenic temperatures, or it can be done through direct contact with liquid nitrogen at −196 °C (−320.8 °F). It is commonly used in the food industry.

Cloud physics is the study of the physical processes that lead to the formation, growth and precipitation of atmospheric clouds. These aerosols are found in the troposphere, stratosphere, and mesosphere, which collectively make up the greatest part of the homosphere. Clouds consist of microscopic droplets of liquid water, tiny crystals of ice, or both, along with microscopic particles of dust, smoke, or other matter, known as condensation nuclei. Cloud droplets initially form by the condensation of water vapor onto condensation nuclei when the supersaturation of air exceeds a critical value according to Köhler theory. Cloud condensation nuclei are necessary for cloud droplets formation because of the Kelvin effect, which describes the change in saturation vapor pressure due to a curved surface. At small radii, the amount of supersaturation needed for condensation to occur is so large, that it does not happen naturally. Raoult's law describes how the vapor pressure is dependent on the amount of solute in a solution. At high concentrations, when the cloud droplets are small, the supersaturation required is smaller than without the presence of a nucleus.

Rime ice forms when supercooled water droplets freeze onto surfaces. In the atmosphere, there are three basic types of rime ice:

Drizzle is a light precipitation which consists of liquid water drops that are smaller than those of rain – generally smaller than 0.5 mm (0.02 in) in diameter. Drizzle is normally produced by low stratiform clouds and stratocumulus clouds. Precipitation rates from drizzle are on the order of a millimetre (0.04 in) per day or less at the ground. Owing to the small size of drizzle drops, under many circumstances drizzle largely evaporates before reaching the surface, and so may be undetected by observers on the ground. The METAR code for drizzle is DZ and for freezing drizzle is FZDZ.

In thermodynamics, nucleation is the first step in the formation of either a new thermodynamic phase or structure via self-assembly or self-organization within a substance or mixture. Nucleation is typically defined to be the process that determines how long an observer has to wait before the new phase or self-organized structure appears. For example, if a volume of water is cooled significantly below 0 °C, it will tend to freeze into ice, but volumes of water cooled only a few degrees below 0 °C often stay completely free of ice for long periods (supercooling). At these conditions, nucleation of ice is either slow or does not occur at all. However, at lower temperatures nucleation is fast, and ice crystals appear after little or no delay.
The Wegener–Bergeron–Findeisen process, is a process of ice crystal growth that occurs in mixed phase clouds in regions where the ambient vapor pressure falls between the saturation vapor pressure over water and the lower saturation vapor pressure over ice. This is a subsaturated environment for liquid water but a supersaturated environment for ice resulting in rapid evaporation of liquid water and rapid ice crystal growth through vapor deposition. If the number density of ice is small compared to liquid water, the ice crystals can grow large enough to fall out of the cloud, melting into rain drops if lower level temperatures are warm enough.

In aeronautics, icing is the formation of water ice on an aircraft. Icing has resulted in numerous fatal accidents in aviation history. Ice accretion and accumulation can affect the external surfaces of an aircraft – in which case it is referred to as airframe icing – or the engine, resulting in carburetor icing, air inlet icing or more generically engine icing. These phenomena may possibly but do not necessarily occur together.

Atmospheric icing occurs in the atmosphere when water droplets suspended in air freeze on objects they come in contact with. It is not the same as freezing rain, which is caused directly by precipitation.

Graupel, also called soft hail or snow pellets, is precipitation that forms when supercooled water droplets in air are collected and freeze on falling snowflakes, forming 2–5 mm (0.08–0.20 in) balls of crisp, opaque rime.

Glaze or glaze ice, also called glazed frost or verglas, is a smooth, transparent and homogeneous ice coating occurring when freezing rain or drizzle hits a surface. It is similar in appearance to clear ice, which forms from supercooled water droplets. It is a relatively common occurrence in temperate climates in the winter when precipitation forms in warm air aloft and falls into below-freezing temperature at the surface.
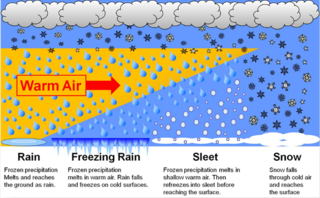
In meteorology, the different types of precipitation often include the character, formation, or phase of the precipitation which is falling to ground level. There are three distinct ways that precipitation can occur. Convective precipitation is generally more intense, and of shorter duration, than stratiform precipitation. Orographic precipitation occurs when moist air is forced upwards over rising terrain and condenses on the slope, such as a mountain.

This glossary of meteorology is a list of terms and concepts relevant to meteorology and atmospheric science, their sub-disciplines, and related fields.














