
The cerebral cortex, also known as the cerebral mantle, is the outer layer of neural tissue of the cerebrum of the brain in humans and other mammals. It is the largest site of neural integration in the central nervous system, and plays a key role in attention, perception, awareness, thought, memory, language, and consciousness. The cerebral cortex is the part of the brain responsible for cognition.
The development of the nervous system, or neural development (neurodevelopment), refers to the processes that generate, shape, and reshape the nervous system of animals, from the earliest stages of embryonic development to adulthood. The field of neural development draws on both neuroscience and developmental biology to describe and provide insight into the cellular and molecular mechanisms by which complex nervous systems develop, from nematodes and fruit flies to mammals.
An apical dendrite is a dendrite that emerges from the apex of a pyramidal cell. Apical dendrites are one of two primary categories of dendrites, and they distinguish the pyramidal cells from spiny stellate cells in the cortices. Pyramidal cells are found in the prefrontal cortex, the hippocampus, the entorhinal cortex, the olfactory cortex, and other areas. Dendrite arbors formed by apical dendrites are the means by which synaptic inputs into a cell are integrated. The apical dendrites in these regions contribute significantly to memory, learning, and sensory associations by modulating the excitatory and inhibitory signals received by the pyramidal cells.
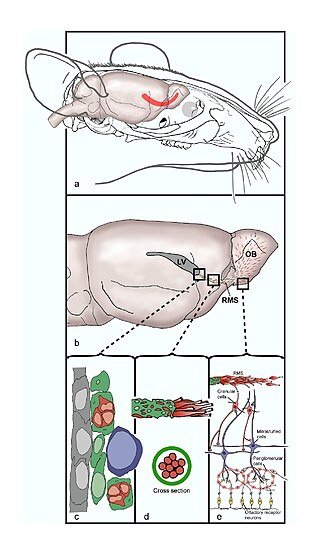
The rostral migratory stream (RMS) is a specialized migratory route found in the brain of some animals along which neuronal precursors that originated in the subventricular zone (SVZ) of the brain migrate to reach the main olfactory bulb (OB). The importance of the RMS lies in its ability to refine and even change an animal's sensitivity to smells, which explains its importance and larger size in the rodent brain as compared to the human brain, as our olfactory sense is not as developed. This pathway has been studied in the rodent, rabbit, and both the squirrel monkey and rhesus monkey. When the neurons reach the OB they differentiate into GABAergic interneurons as they are integrated into either the granule cell layer or periglomerular layer.
Pachygyria is a congenital malformation of the cerebral hemisphere. It results in unusually thick convolutions of the cerebral cortex. Typically, children have developmental delay and seizures, the onset and severity depending on the severity of the cortical malformation. Infantile spasms are common in affected children, as is intractable epilepsy.

The olfactory tubercle (OT), also known as the tuberculum olfactorium, is a multi-sensory processing center that is contained within the olfactory cortex and ventral striatum and plays a role in reward cognition. The OT has also been shown to play a role in locomotor and attentional behaviors, particularly in relation to social and sensory responsiveness, and it may be necessary for behavioral flexibility. The OT is interconnected with numerous brain regions, especially the sensory, arousal, and reward centers, thus making it a potentially critical interface between processing of sensory information and the subsequent behavioral responses.

The islands of Calleja are a group of neural granule cells located within the ventral striatum in the brains of most animals. This region of the brain is part of the limbic system, where it aids in the reinforcing effects of reward-like activities. Within most species, the islands are specifically located within the olfactory tubercle; however, in primates, these islands are located within the nucleus accumbens, the reward center of the brain, since the olfactory tubercle has practically disappeared in the brains of primates. Both of these structures have been implicated in the processing of incentives as well as addictions to drugs. Projections to and from the islands supplement this knowledge with their involvement in the reward pathways for both cocaine and amphetamines.

Radial glial cells, or radial glial progenitor cells (RGPs), are bipolar-shaped progenitor cells that are responsible for producing all of the neurons in the cerebral cortex. RGPs also produce certain lineages of glia, including astrocytes and oligodendrocytes. Their cell bodies (somata) reside in the embryonic ventricular zone, which lies next to the developing ventricular system.

The subventricular zone (SVZ) is a region situated on the outside wall of each lateral ventricle of the vertebrate brain. It is present in both the embryonic and adult brain. In embryonic life, the SVZ refers to a secondary proliferative zone containing neural progenitor cells, which divide to produce neurons in the process of neurogenesis. The primary neural stem cells of the brain and spinal cord, termed radial glial cells, instead reside in the ventricular zone (VZ).

In anatomy of animals, the paleocortex, or paleopallium, is a region within the telencephalon in the vertebrate brain. This type of cortical tissue consists of three cortical laminae. In comparison, the neocortex has six layers and the archicortex has three or four layers. Because the number of laminae that compose a type of cortical tissue seems to be directly proportional to both the information-processing capabilities of that tissue and its phylogenetic age, paleocortex is thought to be an intermediate between the archicortex and the neocortex in both aspects.

Homeobox protein EMX1 is a protein that in humans is encoded by the EMX1 gene. The transcribed EMX1 gene is a member of the EMX family of transcription factors. The EMX1 gene, along with its family members, are expressed in the developing cerebrum. EMX1 plays a role in specification of positional identity, the proliferation of neural stem cells, differentiation of layer-specific neuronal phenotypes and commitment to a neuronal or glial cell fate.

In neuroanatomy, pallium refers to the layers of grey and white matter that cover the upper surface of the cerebrum in vertebrates. The non-pallial part of the telencephalon builds the subpallium. In basal vertebrates, the pallium is a relatively simple three-layered structure, encompassing 3–4 histogenetically distinct domains, plus the olfactory bulb.

T-box, brain, 1 is a transcription factor protein important in vertebrate embryo development. It is encoded by the TBR1 gene. This gene is also known by several other names: T-Brain 1, TBR-1, TES-56, and MGC141978. TBR1 is a member of the TBR1 subfamily of T-box family transcription factors, which share a common DNA-binding domain. Other members of the TBR1 subfamily include EOMES and TBX21. TBR1 is involved in the differentiation and migration of neurons and is required for normal brain development. TBR1 interacts with various genes and proteins in order to regulate cortical development, specifically within layer VI of the developing six-layered human cortex. Studies show that TBR1 may play a role in major neurological diseases such as Alzheimer's disease (AD), Parkinson's disease (PD) and autism spectrum disorder (ASD).
Guidepost cells are cells which assist in the subcellular organization of both neural axon growth and migration. They act as intermediate targets for long and complex axonal growths by creating short and easy pathways, leading axon growth cones towards their target area.

Eomesodermin also known as T-box brain protein 2 (Tbr2) is a protein that in humans is encoded by the EOMES gene.
The development of the cerebral cortex, known as corticogenesis is the process during which the cerebral cortex of the brain is formed as part of the development of the nervous system of mammals including its development in humans. The cortex is the outer layer of the brain and is composed of up to six layers. Neurons formed in the ventricular zone migrate to their final locations in one of the six layers of the cortex. The process occurs from embryonic day 10 to 17 in mice and between gestational weeks seven to 18 in humans.
Cajal–Retzius cells are a heterogeneous population of morphologically and molecularly distinct reelin-producing cells. They are found in the marginal zone/layer I of the developing cerebral cortex and in the immature hippocampus of different species and at different times during embryogenesis and postnatal life.
Neurogenesis is the process by which nervous system cells, the neurons, are produced by neural stem cells (NSCs). This occurs in all species of animals except the porifera (sponges) and placozoans. Types of NSCs include neuroepithelial cells (NECs), radial glial cells (RGCs), basal progenitors (BPs), intermediate neuronal precursors (INPs), subventricular zone astrocytes, and subgranular zone radial astrocytes, among others.
Neurogliaform cells (NGF) are inhibitory (GABAergic) interneurons found in the cortex and the hippocampus. NGF cells represent approximately 10% of the total hippocampal inhibitory interneuron population.

GS homeobox 2 (GSX2) is a protein encoded by a gene of the same name, located on chromosome 4 in humans, and on chromosome 5 in mice.













