Related Research Articles

Cellular respiration is a set of metabolic reactions and processes that take place in the cells of organisms to convert chemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions, which break large molecules into smaller ones, releasing energy. Respiration is one of the key ways a cell releases chemical energy to fuel cellular activity. The overall reaction occurs in a series of biochemical steps, some of which are redox reactions. Although cellular respiration is technically a combustion reaction, it is an unusual one because of the slow, controlled release of energy from the series of reactions.
Anaerobic respiration is respiration using electron acceptors other than molecular oxygen (O2). Although oxygen is not the final electron acceptor, the process still uses a respiratory electron transport chain.

Anammox, an abbreviation for anaerobic ammonium oxidation, is a globally important microbial process of the nitrogen cycle that takes place in many natural environments. The bacteria mediating this process were identified in 1999, and were a great surprise for the scientific community. In the anammox reaction, nitrite and ammonium ions are converted directly into diatomic nitrogen and water.
Methanotrophs are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to survive.

Geobacter is a genus of bacteria. Geobacter species are anaerobic respiration bacterial species which have capabilities that make them useful in bioremediation. Geobacter was found to be the first organism with the ability to oxidize organic compounds and metals, including iron, radioactive metals, and petroleum compounds into environmentally benign carbon dioxide while using iron oxide or other available metals as electron acceptors. Geobacter species are also found to be able to respire upon a graphite electrode. They have been found in anaerobic conditions in soils and aquatic sediment.
Lithotrophs are a diverse group of organisms using an inorganic substrate to obtain reducing equivalents for use in biosynthesis or energy conservation via aerobic or anaerobic respiration. While lithotrophs in the broader sense include photolithotrophs like plants, chemolithotrophs are exclusively microorganisms; no known macrofauna possesses the ability to use inorganic compounds as electron sources. Macrofauna and lithotrophs can form symbiotic relationships, in which case the lithotrophs are called "prokaryotic symbionts". An example of this is chemolithotrophic bacteria in giant tube worms or plastids, which are organelles within plant cells that may have evolved from photolithotrophic cyanobacteria-like organisms. Chemolithotrophs belong to the domains Bacteria and Archaea. The term "lithotroph" was created from the Greek terms 'lithos' (rock) and 'troph' (consumer), meaning "eaters of rock". Many but not all lithoautotrophs are extremophiles.
Microbial metabolism is the means by which a microbe obtains the energy and nutrients it needs to live and reproduce. Microbes use many different types of metabolic strategies and species can often be differentiated from each other based on metabolic characteristics. The specific metabolic properties of a microbe are the major factors in determining that microbe's ecological niche, and often allow for that microbe to be useful in industrial processes or responsible for biogeochemical cycles.
Microbial fuel cell (MFC) is a type of bioelectrochemical fuel cell system that generates electric current by diverting electrons produced from the microbial oxidation of reduced compounds on the anode to oxidized compounds such as oxygen on the cathode through an external electrical circuit. MFCs can be grouped into two general categories: mediated and unmediated. The first MFCs, demonstrated in the early 20th century, used a mediator: a chemical that transfers electrons from the bacteria in the cell to the anode. Unmediated MFCs emerged in the 1970s; in this type of MFC the bacteria typically have electrochemically active redox proteins such as cytochromes on their outer membrane that can transfer electrons directly to the anode. In the 21st century MFCs have started to find commercial use in wastewater treatment.
In biology, syntrophy, synthrophy, or cross-feeding is the phenomenon of one species feeding on the metabolic products of another species. In this type of biological interaction, the growth of one partner depends on the nutrients, growth factors, or substrates provided by the other partner. Jan Dolfing describes syntrophy as "the critical interdependency between producer and consumer". This term for nutritional interdependence is often used in microbiology to describe this symbiotic relationship between bacterial species. Morris et al. have described the process as "obligately mutualistic metabolism".
Electromethanogenesis is a form of electrofuel production where methane is produced by direct biological conversion of electrical current and carbon dioxide.

Bacterial nanowires are electrically conductive appendages produced by a number of bacteria most notably from the Geobacter and Shewanella genera. Conductive nanowires have also been confirmed in the oxygenic cyanobacterium Synechocystis PCC6803 and a thermophilic, methanogenic coculture consisting of Pelotomaculum thermopropionicum and Methanothermobacter thermoautotrophicus. From physiological and functional perspectives, bacterial nanowires are diverse. The precise role microbial nanowires play in their biological systems has not been fully realized, but several proposed functions exist. Outside of a naturally occurring environment, bacterial nanowires have shown potential to be useful in several fields, notably the bioenergy and bioremediation industries.
Rhodopseudomonas palustris is a rod-shaped, Gram-negative purple nonsulfur bacterium, notable for its ability to switch between four different modes of metabolism.
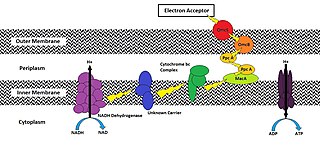
An exoelectrogen normally refers to a microorganism that has the ability to transfer electrons extracellularly. While exoelectrogen is the predominant name, other terms have been used: electrochemically active bacteria, anode respiring bacteria, and electricigens. Electrons exocytosed in this fashion are produced following ATP production using an electron transport chain (ETC) during oxidative phosphorylation. Conventional cellular respiration requires a final electron acceptor to receive these electrons. Cells that use molecular oxygen (O2) as their final electron acceptor are described as using aerobic respiration, while cells that use other soluble compounds as their final electron acceptor are described as using anaerobic respiration. However, the final electron acceptor of an exoelectrogen is found extracellularly and can be a strong oxidizing agent in aqueous solution or a solid conductor/electron acceptor. Two commonly observed acceptors are iron compounds (specifically Fe(III) oxides) and manganese compounds (specifically Mn(III/IV) oxides). As oxygen is a strong oxidizer, cells are able to do this strictly in the absence of oxygen.
Microbial electrosynthesis (MES) is a form of microbial electrocatalysis in which electrons are supplied to living microorganisms via a cathode in an electrochemical cell by applying an electric current. The electrons are then used by the microorganisms to reduce carbon dioxide to yield industrially relevant products. The electric current would ideally be produced by a renewable source of power. This process is the opposite to that employed in a microbial fuel cell, in which microorganisms transfer electrons from the oxidation of compounds to an anode to generate an electric current.
Geothrix fermentans is a rod-shaped, anaerobic bacterium. It is about 0.1 µm in diameter and ranges from 2-3 µm in length. Cell arrangement occurs singly and in chains. Geothrix fermentans can normally be found in aquatic sediments such as in aquifers. As an anaerobic chemoorganotroph, this organism is best known for its ability to use electron acceptors Fe(III), as well as other high potential metals. It also uses a wide range of substrates as electron donors. Research on metal reduction by G. fermentans has contributed to understanding more about the geochemical cycling of metals in the environment.
Geobacter sulfurreducens is a gram-negative metal and sulphur-reducing proteobacterium. It is rod-shaped, obligately anaerobic, non-fermentative, has flagellum and type four pili, and is closely related to Geobacter metallireducens. Geobacter sulfurreducens is an anaerobic species of bacteria that comes from the family of bacteria called Geobacteraceae. Under the genus of Geobacter, G. sulfurreducens is one out of twenty different species. The Geobacter genus was discovered by Derek R. Lovley in 1987. G. sulfurreducens was first isolated in Norman, Oklahoma, USA from materials found around the surface of a contaminated ditch.
Desulfobulbus propionicus is a Gram-negative, anaerobic chemoorganotroph. Three separate strains have been identified: 1pr3T, 2pr4, and 3pr10. It is also the first pure culture example of successful disproportionation of elemental sulfur to sulfate and sulfide. Desulfobulbus propionicus has the potential to produce free energy and chemical products.
Biological photovoltaics (BPV) is an energy-generating technology which uses oxygenic photoautotrophic organisms, or fractions thereof, to harvest light energy and produce electrical power. Biological photovoltaic devices are a type of biological electrochemical system, or microbial fuel cell, and are sometimes also called photo-microbial fuel cells or “living solar cells”. In a biological photovoltaic system, electrons generated by photolysis of water are transferred to an anode. A relatively high-potential reaction takes place at the cathode, and the resulting potential difference drives current through an external circuit to do useful work. It is hoped that using a living organism as the light harvesting material, will make biological photovoltaics a cost-effective alternative to synthetic light-energy-transduction technologies such as silicon-based photovoltaics.
Dissimilatory metal-reducing microorganisms are a group of microorganisms (both bacteria and archaea) that can perform anaerobic respiration utilizing a metal as terminal electron acceptor rather than molecular oxygen (O2), which is the terminal electron acceptor reduced to water (H2O) in aerobic respiration. The most common metals used for this end are iron [Fe(III)] and manganese [Mn(IV)], which are reduced to Fe(II) and Mn(II) respectively, and most microorganisms that reduce Fe(III) can reduce Mn(IV) as well. But other metals and metalloids are also used as terminal electron acceptors, such as vanadium [V(V)], chromium [Cr(VI)], molybdenum [Mo(VI)], cobalt [Co(III)], palladium [Pd(II)], gold [Au(III)], and mercury [Hg(II)].

Microbial oxidation of sulfur is the oxidation of sulfur by microorganisms to build their structural components. The oxidation of inorganic compounds is the strategy primarily used by chemolithotrophic microorganisms to obtain energy to survive, grow and reproduce. Some inorganic forms of reduced sulfur, mainly sulfide (H2S/HS−) and elemental sulfur (S0), can be oxidized by chemolithotrophic sulfur-oxidizing prokaryotes, usually coupled to the reduction of oxygen (O2) or nitrate (NO3−). Anaerobic sulfur oxidizers include photolithoautotrophs that obtain their energy from sunlight, hydrogen from sulfide, and carbon from carbon dioxide (CO2).
References
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 Holmes DE, Nicoll JS, Bond DR, Lovley DR (October 2004). "Potential role of a novel psychrotolerant member of the family Geobacteraceae, Geopsychrobacter electrodiphilus gen. nov., sp. nov., in electricity production by a marine sediment fuel cell". Applied and Environmental Microbiology. 70 (10): 6023–30. Bibcode:2004ApEnM..70.6023H. doi:10.1128/AEM.70.10.6023-6030.2004. PMC 522133 . PMID 15466546.
- ↑ Holmes DE, Rotaru AE, Ueki T, Shrestha PM, Ferry JG, Lovley DR (2018). "Electron and Proton Flux for Carbon Dioxide Reduction in Methanosarcina barkeri During Direct Interspecies Electron Transfer". Frontiers in Microbiology. 9: 3109. doi: 10.3389/fmicb.2018.03109 . PMC 6315138 . PMID 30631315.
- ↑ Zeng GQ, Jia XS, Zheng XH, Yang LP, Sun GP (November 2014). "[Analysis of microbial community variation in the domestication process of sludge in a sulfate-reducing reactor]". Huan Jing Ke Xue = Huanjing Kexue. 35 (11): 4244–50. PMID 25639102.
- ↑ Mikucki JA, Priscu JC (June 2007). "Bacterial diversity associated with Blood Falls, a subglacial outflow from the Taylor Glacier, Antarctica". Applied and Environmental Microbiology. 73 (12): 4029–39. Bibcode:2007ApEnM..73.4029M. doi:10.1128/AEM.01396-06. PMC 1932727 . PMID 17468282.
- ↑ Mikucki JA, Pearson A, Johnston DT, Turchyn AV, Farquhar J, Schrag DP, Anbar AD, Priscu JC, Lee PA (April 2009). "A contemporary microbially maintained subglacial ferrous "ocean"". Science. 324 (5925): 397–400. Bibcode:2009Sci...324..397M. doi:10.1126/science.1167350. PMID 19372431. S2CID 44802632.
- ↑ Bond DR, Lovley DR (March 2003). "Electricity production by Geobacter sulfurreducens attached to electrodes". Applied and Environmental Microbiology. 69 (3): 1548–55. Bibcode:2003ApEnM..69.1548B. doi:10.1128/AEM.69.3.1548-1555.2003. PMC 150094 . PMID 12620842.
- ↑ Tender LM, Reimers CE, Stecher HA, Holmes DE, Bond DR, Lowy DA, Pilobello K, Fertig SJ, Lovley DR (August 2002). "Harnessing microbially generated power on the seafloor". Nature Biotechnology. 20 (8): 821–5. doi:10.1038/nbt716. PMID 12091916. S2CID 927966.
- ↑ Reimers CE, Tender LM, Fertig S, Wang W (January 2001). "Harvesting energy from the marine sediment--water interface". Environmental Science & Technology. 35 (1): 192–5. Bibcode:2001EnST...35..192R. doi:10.1021/es001223s. PMID 11352010.