
Terpenes are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predominantly by plants, particularly conifers. Terpenes are further classified by the number of carbons: monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), as examples. The terpene alpha-pinene is a major component of the common solvent, turpentine.

The mevalonate pathway, also known as the isoprenoid pathway or HMG-CoA reductase pathway is an essential metabolic pathway present in eukaryotes, archaea, and some bacteria. The pathway produces two five-carbon building blocks called isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP), which are used to make isoprenoids, a diverse class of over 30,000 biomolecules such as cholesterol, vitamin K, coenzyme Q10, and all steroid hormones.

Dimethylallyl pyrophosphate is an isoprenoid precursor. It is a product of both the mevalonate pathway and the MEP pathway of isoprenoid precursor biosynthesis. It is an isomer of isopentenyl pyrophosphate (IPP) and exists in virtually all life forms. The enzyme isopentenyl pyrophosphate isomerase catalyzes isomerization between DMAPP and IPP.

Sabinene is a natural bicyclic monoterpene with the molecular formula C10H16. It is isolated from the essential oils of a variety of plants including Marjoram, holm oak (Quercus ilex) and Norway spruce (Picea abies). It has a strained ring system with a cyclopentane ring fused to a cyclopropane ring.

Isopentenyl pyrophosphate is an isoprenoid precursor. IPP is an intermediate in the classical, HMG-CoA reductase pathway and in the non-mevalonate MEP pathway of isoprenoid precursor biosynthesis. Isoprenoid precursors such as IPP, and its isomer DMAPP, are used by organisms in the biosynthesis of terpenes and terpenoids.
Farnesyl pyrophosphate (FPP), also known as farnesyl diphosphate (FDP), is an intermediate in the biosynthesis of terpenes and terpenoids such as sterols and carotenoids. It is also used in the synthesis of CoQ, as well as dehydrodolichol diphosphate.
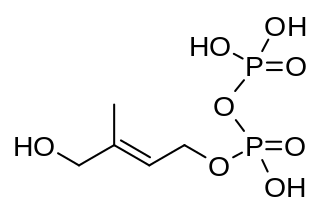
(E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate (HMBPP or HMB-PP) is an intermediate of the MEP pathway (non-mevalonate pathway) of isoprenoid biosynthesis. The enzyme HMB-PP synthase (GcpE, IspG) catalyzes the conversion of 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MEcPP) into HMB-PP. HMB-PP is then converted further to isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) by HMB-PP reductase (LytB, IspH).
The non-mevalonate pathway—also appearing as the mevalonate-independent pathway and the 2-C-methyl-D-erythritol 4-phosphate/1-deoxy-D-xylulose 5-phosphate (MEP/DOXP) pathway—is an alternative metabolic pathway for the biosynthesis of the isoprenoid precursors isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP). The currently preferred name for this pathway is the MEP pathway, since MEP is the first committed metabolite on the route to IPP.

Andrographolide is a labdane diterpenoid that has been isolated from the stem and leaves of Andrographis paniculata. Andrographolide is an extremely bitter substance.

Squalene synthase (SQS) or farnesyl-diphosphate:farnesyl-diphosphate farnesyl transferase is an enzyme localized to the membrane of the endoplasmic reticulum. SQS participates in the isoprenoid biosynthetic pathway, catalyzing a two-step reaction in which two identical molecules of farnesyl pyrophosphate (FPP) are converted into squalene, with the consumption of NADPH. Catalysis by SQS is the first committed step in sterol synthesis, since the squalene produced is converted exclusively into various sterols, such as cholesterol, via a complex, multi-step pathway. SQS belongs to squalene/phytoene synthase family of proteins.

Isopentenyl pyrophosphate isomerase, also known as Isopentenyl-diphosphate delta isomerase, is an isomerase that catalyzes the conversion of the relatively un-reactive isopentenyl pyrophosphate (IPP) to the more-reactive electrophile dimethylallyl pyrophosphate (DMAPP). This isomerization is a key step in the biosynthesis of isoprenoids through the mevalonate pathway and the MEP pathway.

Dimethylallyltranstransferase (DMATT), also known as farnesylpyrophosphate synthase (FPPS) or as farnesyldiphosphate synthase (FDPS), is an enzyme that in humans is encoded by the FDPS gene and catalyzes the transformation of dimethylallylpyrophosphate (DMAPP) and isopentenyl pyrophosphate (IPP) into farnesylpyrophosphate (FPP).

Juvabione, historically known as the paper factor, is the methyl ester of todomatuic acid. Both are sesquiterpenes (C15) found in the wood of true firs of the genus Abies. They occur naturally as part of a mixture of sesquiterpenes based upon the bisabolane scaffold. Sesquiterpenes of this family are known as insect juvenile hormone analogues (IJHA) because of their ability to mimic juvenile activity in order to stifle insect reproduction and growth. These compounds play important roles in conifers as the second line of defense against insect induced trauma and fungal pathogens.

Polyprenyl synthetases are a class of enzymes responsible for synthesis of isoprenoids. Isoprenoid compounds are synthesized by various organisms. For example, in eukaryotes the isoprenoid biosynthetic pathway is responsible for the synthesis of a variety of end products including cholesterol, dolichol, ubiquinone or coenzyme Q. In bacteria this pathway leads to the synthesis of isopentenyl tRNA, isoprenoid quinones, and sugar carrier lipids. Among the enzymes that participate in that pathway, are a number of polyprenyl synthetase enzymes which catalyze a 1'4-condensation between 5-carbon isoprene units. It has been shown that all the above enzymes share some regions of sequence similarity. Two of these regions are rich in aspartic-acid residues and could be involved in the catalytic mechanism and/or the binding of the substrates.
All-trans-nonaprenyl-diphosphate synthase is an enzyme with systematic name geranyl-diphosphate:isopentenyl-diphosphate transtransferase . This enzyme catalyses the following chemical reaction

All-trans-octaprenyl-diphosphate synthase is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate:isopentenyl-diphosphate farnesyltranstransferase . This enzyme catalyses the following chemical reaction
All-trans-decaprenyl-diphosphate synthase is an enzyme with systematic name (2E,6E)-farnesyl-diphosphate:isopentenyl-diphosphate farnesyltranstransferase . This enzyme catalyses the following chemical reaction
(2Z,6Z)-farnesyl diphosphate synthase is an enzyme with systematic name dimethylallyl-diphosphate:isopentenyl-diphosphate cistransferase . This enzyme catalyses the following chemical reaction
The squalene/phytoene synthase family represents proteins that catalyze the head-to-head condensation of C15 and C20 prenyl units (i.e. farnesyl diphosphate and genranylgeranyl diphosphate). This enzymatic step constitutes part of steroid and carotenoid biosynthesis pathway. Squalene synthase EC (SQS) and Phytoene synthase EC (PSY) are two well-known examples of this protein family and share a number of functional similarities. These similarities are also reflected in their primary structure. In particular three well conserved regions are shared by SQS and PSY; they could be involved in substrate binding and/or the catalytic mechanism. SQS catalyzes the conversion of two molecules of farnesyl diphosphate (FPP) into squalene. It is the first committed step in the cholesterol biosynthetic pathway. The reaction carried out by SQS is catalyzed in two separate steps: the first is a head-to-head condensation of the two molecules of FPP to form presqualene diphosphate; this intermediate is then rearranged in a NADP-dependent reduction, to form squalene:

Drimentine G belongs to the family of drimentines, which are terpenylated diketopiperazines. As the name suggests, DMT G contains two different parts, one comes from the non-ribosomal peptide synthetase (NRPS) pathway to generate the diketopiperazine ring structure. The other part comes from either the mevalonic acid pathway (MVA) or deoxy xylulose phosphate pathway (MEP) to produce sesquiterpenes needed for interaction with the diketopiperazine. This molecule is said to be useful as an antibiotic to treat bacterial or fungi infections, has therapeutic application to treat animal health, and can serve as a pest control for plants.
















