The enzyme Uridine monophosphate synthase catalyses the formation of uridine monophosphate (UMP), an energy-carrying molecule in many important biosynthetic pathways. In humans, the gene that codes for this enzyme is located on the long arm of chromosome 3 (3q13).
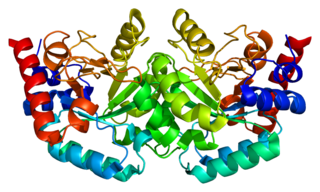
Tryptophan synthase or tryptophan synthetase is an enzyme that catalyses the final two steps in the biosynthesis of tryptophan. It is commonly found in Eubacteria, Archaebacteria, Protista, Fungi, and Plantae. However, it is absent from Animalia. It is typically found as an α2β2 tetramer. The α subunits catalyze the reversible formation of indole and glyceraldehyde-3-phosphate (G3P) from indole-3-glycerol phosphate (IGP). The β subunits catalyze the irreversible condensation of indole and serine to form tryptophan in a pyridoxal phosphate (PLP) dependent reaction. Each α active site is connected to a β active site by a 25 angstrom long hydrophobic channel contained within the enzyme. This facilitates the diffusion of indole formed at α active sites directly to β active sites in a process known as substrate channeling. The active sites of tryptophan synthase are allosterically coupled.

Aromatic L-amino acid decarboxylase, also known as DOPA decarboxylase (DDC), tryptophan decarboxylase, and 5-hydroxytryptophan decarboxylase, is a lyase enzyme, located in region 7p12.2-p12.1.

Malonyl-CoA is a coenzyme A derivative of malonic acid.
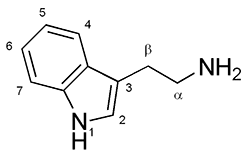
Indolamines are a family of neurotransmitters that share a common molecular structure. Indolamines are a classification of monoamine neurotransmitter, along with catecholamines and ethylamine derivatives. A common example of an indolamine is the tryptophan derivative serotonin, a neurotransmitter involved in mood and sleep. Another example of an indolamine is melatonin.
The enzyme histidine decarboxylase is transcribed on chromosome 15, region q21.1-21.2, and catalyzes the decarboxylation of histidine to form histamine. In mammals, histamine is an important biogenic amine with regulatory roles in neurotransmission, gastric acid secretion and immune response. Histidine decarboxylase is the sole member of the histamine synthesis pathway, producing histamine in a one-step reaction. Histamine cannot be generated by any other known enzyme. HDC is therefore the primary source of histamine in most mammals and eukaryotes. The enzyme employs a pyridoxal 5'-phosphate (PLP) cofactor, in similarity to many amino acid decarboxylases. Eukaryotes, as well as gram-negative bacteria share a common HDC, while gram-positive bacteria employ an evolutionarily unrelated pyruvoyl-dependent HDC. In humans, histidine decarboxylase is encoded by the HDC gene.
In enzymology, an oxaloglycolate reductase (decarboxylating) (EC 1.1.1.92) is an enzyme that catalyzes the chemical reaction
In enzymology, a sterol-4alpha-carboxylate 3-dehydrogenase (decarboxylating) (EC 1.1.1.170) is an enzyme that catalyzes the chemical reaction

Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the bark and stem of Camptotheca acuminata, a tree native to China used in traditional Chinese medicine. It has been used clinically more recently in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT analogues have been approved and are used in cancer chemotherapy today: topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin has also been found in other plants including Chonemorpha fragrans.
The enzyme 3-hydroxy-2-methylpyridine-4,5-dicarboxylate 4-decarboxylase (EC 4.1.1.51) catalyzes the chemical reaction

The enzyme Acid-Induced Arginine Decarboxylase (AdiA), also commonly referred to as arginine decarboxylase, catalyzes the conversion of L-arginine into agmatine and carbon dioxide. The process consumes a proton in the decarboxylation and employs a pyridoxal-5'-phosphate (PLP) cofactor, similar to other enzymes involved in amino acid metabolism, such as ornithine decarboxylase and glutamine decarboxylase. It is found in bacteria and virus, though most research has so far focused on forms of the enzyme in bacteria. During the AdiA catalyzed decarboxylation of arginine, the necessary proton is consumed from the cell cytoplasm which helps to prevent the over-accumulation of protons inside the cell and serves to increase the intracellular pH. Arginine decarboxylase is part of an enzymatic system in Escherichia coli, Salmonella Typhimurium, and methane-producing bacteria Methanococcus jannaschii that makes these organisms acid resistant and allows them to survive under highly acidic medium.
The enzyme indolepyruvate decarboxylase (EC 4.1.1.74) catalyzes the chemical reaction
The enzyme uracil-5-carboxylate decarboxylase (EC 4.1.1.66) catalyzes the chemical reaction
In enzymology, a hydroxyisourate hydrolase (EC 3.5.2.17) is an enzyme that catalyzes the chemical reaction

Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure. It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan. It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.
2-oxoglutarate/L-arginine monooxygenase/decarboxylase (succinate-forming) (EC 1.14.11.34, ethylene-forming enzyme, EFE) is an enzyme with systematic name L-arginine,2-oxoglutarate:oxygen oxidoreductase (succinate-forming). This enzyme catalyses the following chemical reaction
Pyrrole-2-carboxylate decarboxylase (EC 4.1.1.93) is an enzyme with systematic name pyrrole-2-carboxylate carboxy-lyase. This enzyme catalyses the following chemical reaction
Indoleacetate decarboxylase (IAD) is a glycyl radical enzyme that catalyses the decarboxylation of indoleacetate to form skatole, which is a malodorous organic compound that gives animal faeces their characteristic smell. This decarboxylation is the last step of the tryptophan fermentation in some types of anaerobic bacteria.

L-Tryptophan decarboxylase is an enzyme distinguished by the substrate L-tryptophan.










