Glycoproteins are proteins which contain oligosaccharide (sugar) chains covalently attached to amino acid side-chains. The carbohydrate is attached to the protein in a cotranslational or posttranslational modification. This process is known as glycosylation. Secreted extracellular proteins are often glycosylated.
A congenital disorder of glycosylation is one of several rare inborn errors of metabolism in which glycosylation of a variety of tissue proteins and/or lipids is deficient or defective. Congenital disorders of glycosylation are sometimes known as CDG syndromes. They often cause serious, sometimes fatal, malfunction of several different organ systems in affected infants. The most common sub-type is PMM2-CDG where the genetic defect leads to the loss of phosphomannomutase 2 (PMM2), the enzyme responsible for the conversion of mannose-6-phosphate into mannose-1-phosphate.

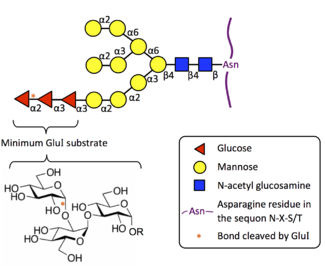
Oligosaccharyltransferase or OST (EC 2.4.1.119) is a membrane protein complex that transfers a 14-sugar oligosaccharide from dolichol to nascent protein. It is a type of glycosyltransferase. The sugar Glc3Man9GlcNAc2 (where Glc=Glucose, Man=Mannose, and GlcNAc=N-acetylglucosamine) is attached to an asparagine (Asn) residue in the sequence Asn-X-Ser or Asn-X-Thr where X is any amino acid except proline. This sequence is called a glycosylation sequon. The reaction catalyzed by OST is the central step in the N-linked glycosylation pathway.

α-Glucosidase (EC 3.2.1.20, is a glucosidase located in the brush border of the small intestine that acts upon α bonds:
The enzyme mannosyl-glycoprotein endo-β-N-acetylglucosaminidase (endoglycosidase H) (EC 3.2.1.96) has systematic name glycopeptide-D-mannosyl-N4-(N-acetyl-D-glucosaminyl)2-asparagine 1,4-N-acetyl-β-glucosaminohydrolase. It is a highly specific endoglycosidase which cleaves asparagine-linked mannose rich oligosaccharides, but not highly processed complex oligosaccharides from glycoproteins. It is used for research purposes to deglycosylate glycoproteins and to monitor intracellular protein trafficking through the secretory pathway.

Neutral alpha-glucosidase C is an enzyme that in humans is encoded by the GANC gene.

Mannosyl-oligosaccharide glucosidase is an enzyme that in humans is encoded by the MOGS gene.
N-linked glycosylation is the attachment of an oligosaccharide, a carbohydrate consisting of several sugar molecules, sometimes also referred to as glycan, to a nitrogen atom, in a process called N-glycosylation, studied in biochemistry. The resulting protein is called an N-linked glycan, or simply an N-glycan.
Alpha-1,3-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-D-glucosamine:3-(alpha-D-mannosyl)-beta-D-mannosyl-glycoprotein 2-beta-N-acetyl-D-glucosaminyltransferase. This enzyme catalyses the following chemical reaction
Alpha-1,6-mannosyl-glycoprotein 2-beta-N-acetylglucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-D-glucosamine:6-(alpha-D-mannosyl)-beta-D-mannosyl-glycoprotein 2-beta-N-acetyl-D-glucosaminyltransferase. This enzyme catalyses the following chemical reaction
Alpha-1,6-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase is an enzyme with systematic name UDP-N-acetyl-D-glucosamine:2,6-bis(N-acetyl-beta-D-glucosaminyl)-alpha-D-mannosyl-glycoprotein 4-beta-N-acetyl-D-glucosaminyltransferase. This enzyme catalyses the following chemical reaction
GDP-Man:Man2GlcNAc2-PP-dolichol alpha-1,6-mannosyltransferase is an enzyme with systematic name GDP-D-mannose:D-Man-alpha-(1->3)-D-Man-beta-(1->4)-D-GlcNAc-beta-(1->4)-D-GlcNAc-diphosphodolichol alpha-6-mannosyltransferase. This enzyme catalyses the following chemical reaction
Dolichyl-P-Man:Man5GlcNAc2-PP-dolichol alpha-1,3-mannosyltransferase is an enzyme with systematic name dolichyl beta-D-mannosyl phosphate:D-Man-alpha-(1->2)-D-Man-alpha-(1->2)-D-Man-alpha-(1->3)-(D-Man-alpha- )-D-Man-beta-(1->4)-D-GlcNAc-beta-(1->4)-D-GlcNAc-diphosphodolichol alpha-1,3-mannosyltransferase. This enzyme catalyses the following chemical reaction

Dolichyl-P-Glc:Glc1Man9GlcNAc2-PP-dolichol alpha-1,3-glucosyltransferase is an enzyme with systematic name dolichyl beta-D-glucosyl phosphate:D-Glc-alpha-(1->3)-D-Man-alpha-(1->2)-D-Man-alpha-(1->2)-D-Man-alpha-(1->3)-(D-Man-alpha- -D-Man-alpha- - -D-Man-alpha- )-D-Man-beta-(1->4)-D-GlcNAc-beta-(1->4)-D-GlcNAc-diphosphodolichol alpha-1,3-glucosyltransferase.
Mannosyl-oligosaccharide 1,2-α-mannosidase (EC 3.2.1.113, mannosidase 1A, mannosidase 1B, 1,2-α-mannosidase, exo-α-1,2-mannanase, mannose-9 processing α-mannosidase, glycoprotein processing mannosidase I, mannosidase I, Man9-mannosidase, ManI, 1,2-α-mannosyl-oligosaccharide α-D-mannohydrolase) is an enzyme with systematic name 2-α-mannosyl-oligosaccharide α-D-mannohydrolase. It catalyses the hydrolysis of the terminal (1→2)-linked α-D-mannose residues in the oligo-mannose oligosaccharide Man9(GlcNAc)2.
Mannosyl-oligosaccharide 1,3-1,6-α-mannosidase, also known as Golgi α-mannosidase II, is an enzyme with systematic name (1→3)-(1→6)-mannosyl-oligosaccharide α-D-mannohydrolase. It catalyses the hydrolysis of the terminal (1→3)- and (1→6)-linked α-D-mannose residues in the mannosyl-oligosaccharide Man5(GlcNAc)3.
Glycoprotein endo-α-1,2-mannosidase (EC 3.2.1.130, glucosylmannosidase, endo-α-D-mannosidase, endo-α-mannosidase, endomannosidase, glucosyl mannosidase) is an enzyme with systematic name glycoprotein glucosylmannohydrolase. It catalyses the hydrolysis of the terminal α-D-glucosyl-(1,3)-D-mannosyl unit from the GlcMan9(GlcNAc)2 oligosaccharide component of the glycoprotein produced in the Golgi membrane.
Mannosylglycoprotein endo-beta-mannosidase is an enzyme. This enzyme catalyses the following chemical reaction:

Peptide:N-glycosidase F, commonly referred to as PNGase F, is an amidase of the peptide-N4-(N-acetyl-beta-glucosaminyl)asparagine amidase class. PNGase F works by cleaving between the innermost GlcNAc and asparagine residues of high mannose, hybrid, and complex oligosaccharides from N-linked glycoproteins and glycopeptides. This results in a deaminated protein or peptide and a free glycan.
Armando J. Parodi is an Argentine glycobiologist. He did his initial education at the School of Sciences of the University of Buenos Aires. His PhD work was done under Luis Federico Leloir, a recipient of the Nobel Prize in Chemistry for his work involving the finding of sugar nucleotides and how they play a role in the making of oligosaccharides and polysaccharides. He also pursued postdoc work at the Pasteur Institute in Paris, France and Duke University in Durham, NC, USA.