
A neurotransmitter is a signaling molecule secreted by a neuron to affect another cell across a synapse. The cell receiving the signal, or target cell, may be another neuron, but could also be a gland or muscle cell.

Serotonin or 5-hydroxytryptamine (5-HT) is a monoamine neurotransmitter. Its biological function is complex, touching on diverse functions including mood, cognition, reward, learning, memory, and numerous physiological processes such as vomiting and vasoconstriction.

The raphe nuclei are a moderate-size cluster of nuclei found in the brain stem. They have 5-HT1 receptors which are coupled with Gi/Go-protein-inhibiting adenyl cyclase. They function as autoreceptors in the brain and decrease the release of serotonin. The anxiolytic drug Buspirone acts as partial agonist against these receptors. Selective serotonin reuptake inhibitor (SSRI) antidepressants are believed to act in these nuclei, as well as at their targets.

The ventral tegmental area (VTA), also known as the ventral tegmental area of Tsai, or simply ventral tegmentum, is a group of neurons located close to the midline on the floor of the midbrain. The VTA is the origin of the dopaminergic cell bodies of the mesocorticolimbic dopamine system and other dopamine pathways; it is widely implicated in the drug and natural reward circuitry of the brain. The VTA plays an important role in a number of processes, including reward cognition and orgasm, among others, as well as several psychiatric disorders. Neurons in the VTA project to numerous areas of the brain, ranging from the prefrontal cortex to the caudal brainstem and several regions in between.

5-Hydroxytryptophan (5-HTP), used medically as oxitriptan, is a naturally occurring amino acid and chemical precursor as well as a metabolic intermediate in the biosynthesis of the neurotransmitter serotonin.

In neuroanatomy, the pretectal area, or pretectum, is a midbrain structure composed of seven nuclei and comprises part of the subcortical visual system. Through reciprocal bilateral projections from the retina, it is involved primarily in mediating behavioral responses to acute changes in ambient light such as the pupillary light reflex, the optokinetic reflex, and temporary changes to the circadian rhythm. In addition to the pretectum's role in the visual system, the anterior pretectal nucleus has been found to mediate somatosensory and nociceptive information.

The reticular formation is a set of interconnected nuclei in the brainstem that spans from the lower end of the medulla oblongata to the upper end of the midbrain. The neurons of the reticular formation make up a complex set of neural networks in the core of the brainstem. The reticular formation is made up of a diffuse net-like formation of reticular nuclei which is not well-defined. It may be seen as being made up of all the interspersed cells in the brainstem between the more compact and named structures.

The habenula is a small bilateral neuronal structure in the brain of vertebrates, that has also been called a microstructure since it is no bigger than a pea. The naming as little rein describes its elongated shape in the epithalamus, where it borders the third ventricle, and lies in front of the pineal gland.

The septal area, consisting of the lateral septum and medial septum, is an area in the lower, posterior part of the medial surface of the frontal lobe, and refers to the nearby septum pellucidum.
The dorsal longitudinal fasciculus (DLF) is a distinctive nerve tract in the midbrain. It extends from the hypothalamus rostrally to the spinal cord caudally, and contains both descending and ascending fibers.

The dorsal raphe nucleus is one of the raphe nuclei. It is situated in the brainstem at the midline. It has rostral and caudal subdivisions:
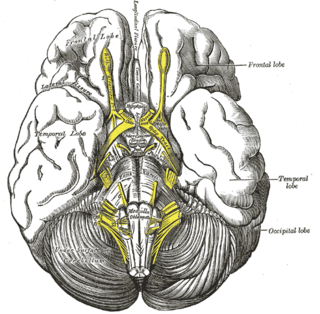
The diagonal band of Broca interconnects the amygdala and the septal area. It is one of the olfactory structures. It is situated upon the inferior aspect of the brain. It forms the medial margin of the anterior perforated substance.

Tryptophan hydroxylase 2 (TPH2) is an isozyme of tryptophan hydroxylase found in vertebrates. In humans, TPH2 is primarily expressed in the serotonergic neurons of the brain, with the highest expression in the raphe nucleus of the midbrain. Until the discovery of TPH2 in 2003, serotonin levels in the central nervous system were believed to be regulated by serotonin synthesis in peripheral tissues, in which tryptophan hydroxylase is the dominant form.

Hippocampus anatomy describes the physical aspects and properties of the hippocampus, a neural structure in the medial temporal lobe of the brain. It has a distinctive, curved shape that has been likened to the sea-horse monster of Greek mythology and the ram's horns of Amun in Egyptian mythology. This general layout holds across the full range of mammalian species, from hedgehog to human, although the details vary. For example, in the rat, the two hippocampi look similar to a pair of bananas, joined at the stems. In the human and other primates, the portion of the hippocampus near the base of the temporal lobe is much broader than the part at the top. Due to the three-dimensional curvature of this structure, two-dimensional sections such as shown are commonly seen. Neuroimaging pictures can show a number of different shapes, depending on the angle and location of the cut.

In neuroanatomy, pallium refers to the layers of grey and white matter that cover the upper surface of the cerebrum in vertebrates. The non-pallial part of the telencephalon builds the subpallium. In basal vertebrates, the pallium is a relatively simple three-layered structure, encompassing 3–4 histogenetically distinct domains, plus the olfactory bulb.
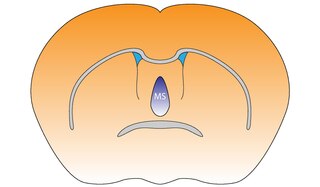
The medial septal nucleus (MS) is one of the septal nuclei. Neurons in this nucleus give rise to the bulk of efferents from the septal nuclei. A major projection from the medial septal nucleus terminates in the hippocampal formation.
Sleep onset is the transition from wakefulness into sleep. Sleep onset usually transits into non-rapid eye movement sleep but under certain circumstances it is possible to transit from wakefulness directly into rapid eye movement sleep.
Serotonergic cell groups refer to collections of neurons in the central nervous system that have been demonstrated by histochemical fluorescence to contain the neurotransmitter serotonin (5-hydroxytryptamine). Since they are for the most part localized to classical brainstem nuclei, particularly the raphe nuclei, they are more often referred to by the names of those nuclei than by the B1-9 nomenclature. These cells appear to be common across most mammals and have two main regions in which they develop; one forms in the mesencephlon and the rostral pons and the other in the medulla oblongata and the caudal pons.

The parabrachial nuclei, also known as the parabrachial complex, are a group of nuclei in the dorsolateral pons that surrounds the superior cerebellar peduncle as it enters the brainstem from the cerebellum. They are named from the Latin term for the superior cerebellar peduncle, the brachium conjunctivum. In the human brain, the expansion of the superior cerebellar peduncle expands the parabrachial nuclei, which form a thin strip of grey matter over most of the peduncle. The parabrachial nuclei are typically divided along the lines suggested by Baxter and Olszewski in humans, into a medial parabrachial nucleus and lateral parabrachial nucleus. These have in turn been subdivided into a dozen subnuclei: the superior, dorsal, ventral, internal, external and extreme lateral subnuclei; the lateral crescent and subparabrachial nucleus along the ventrolateral margin of the lateral parabrachial complex; and the medial and external medial subnuclei
















