Related Research Articles
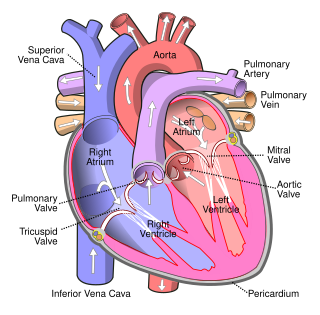
The pulmonary veins are the veins that transfer oxygenated blood from the lungs to the heart. The largest pulmonary veins are the four main pulmonary veins, two from each lung that drain into the left atrium of the heart. The pulmonary veins are part of the pulmonary circulation.

Catheter ablation is a procedure that uses radio-frequency energy or other sources to terminate or modify a faulty electrical pathway from sections of the heart of those who are prone to developing cardiac arrhythmias such as atrial fibrillation, atrial flutter and Wolff-Parkinson-White syndrome. If not controlled, such arrhythmias increase the risk of ventricular fibrillation and sudden cardiac arrest. The ablation procedure can be classified by energy source: radiofrequency ablation and cryoablation.

Radiofrequency ablation (RFA), also called fulguration, is a medical procedure in which part of the electrical conduction system of the heart, tumor or other dysfunctional tissue is ablated using the heat generated from medium frequency alternating current. RFA is generally conducted in the outpatient setting, using either local anesthetics or twilight anesthesia. When it is delivered via catheter, it is called radiofrequency catheter ablation.

Aortic valve repair or aortic valve reconstruction is the reconstruction of both form and function of a dysfunctional aortic valve. Most frequently it is used for the treatment of aortic regurgitation. It can also become necessary for the treatment of aortic aneurysm, less frequently for congenital aortic stenosis.
Cor triatriatum is a congenital heart defect where the left atrium or right atrium is subdivided by a thin membrane, resulting in three atrial chambers.
Arterial switch operation (ASO) or arterial switch, is an open heart surgical procedure used to correct dextro-transposition of the great arteries (d-TGA).
The Dor procedure is a medical technique used as part of heart surgery and originally introduced by the French cardiac surgeon Vincent Dor (b.1932). It is also known as endoventricular circular patch plasty (EVCPP).
The Kawashima procedure is used for congenital heart disease with a single effective ventricle and an interrupted inferior vena cava (IVC). It was first performed in 1978 and reported in 1984.
The Cox maze procedure, also known as maze procedure, is a type of heart surgery for atrial fibrillation.
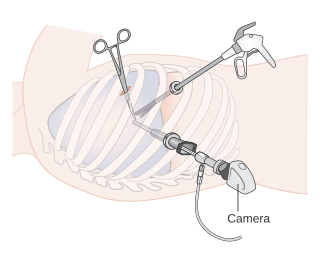
Video-assisted thoracoscopic surgery (VATS) is a type of minimally invasive thoracic surgery performed using a small video camera mounted to a fiberoptic thoracoscope, with or without angulated visualization, which allows the surgeon to see inside the chest by viewing the video images relayed onto a television screen, and perform procedures using elongated surgical instruments. The camera and instruments are inserted into the patient's chest cavity through small incisions in the chest wall, usually via specially designed guiding tubes known as "ports".
The Sensei X robotic catheter is a medical robot designed to enhance a physician’s ability to perform complex operations using a small flexible tube called a catheter. As open surgical procedures that require large incisions have given way to minimally invasive surgeries in which the surgeon gains access to the target organs through small incisions using specialized surgical tools. One important tool used in many of these procedures is a catheter used to deliver many of things a surgeon needs to do his work, to impact target tissue and deliver a variety of medicines or disinfecting agents to treat disease or infection.

Atrial fibrillation is an abnormal heart rhythm (arrhythmia) characterized by rapid and irregular beating of the atrial chambers of the heart. It often begins as short periods of abnormal beating, which become longer or continuous over time. It may also start as other forms of arrhythmia such as atrial flutter that then transform into AF.

Anomalous pulmonary venous connection is a congenital heart defect of the pulmonary veins. It can be a total anomalous pulmonary venous connection, wherein all four pulmonary veins are incorrectly positioned, or a partial anomalous pulmonary venous connection, wherein only some of the pulmonary veins are incorrectly positioned.

Left atrial appendage occlusion (LAAO), also referred to as left atrial appendage closure (LAAC), is a procedure used to reduce the risk of blood clots from the left atrial appendage entering the bloodstream and causing a stroke in those with non-valvular atrial fibrillation.
The management of atrial fibrillation (AF) is focused on preventing temporary circulatory instability, stroke and other ischemic events. Control of heart rate and rhythm are principally used to achieve the former, while anticoagulation may be employed to decrease the risk of stroke. Within the context of stroke, the discipline may be referred to as stroke prevention in atrial fibrillation (SPAF). In emergencies, when circulatory collapse is imminent due to uncontrolled rapid heart rate, immediate cardioversion may be indicated.
James L. Cox is an American cardiothoracic surgeon and medical innovator best known for the development of the Cox maze procedure for treatment of atrial fibrillation in 1987.
An Af-nest or Atrial Fibrillation Nest (AFN) is a locus or cluster in the atrial wall with distinct electrical features and properties originated by fibrillar myocardium. It plays as an "electrical multiplier" re-feeding the atrial fibrillation.

Topera, Inc. is a cardiac arrhythmia mapping company for targeting catheter ablation company launched in San Diego, California and specializes in mapping electrical signals of the heart. Topera's headquarters are located in Palo Alto, California. The company uses 3D analysis and mapping to detect the sources of atrial fibrillation, atrial flutter, and atrial tachycardia and ventricular tachycardia to identify targets for catheter ablation.
Yaariv Khaykin is a Canadian cardiologist and a clinical researcher in the area of electrophysiology. He is the director of the Newmarket Electrophysiology Research Group at the Southlake Regional Health Centre. He has published research into complex ablation and pioneered cardiac ablation methods.
Ganglionated plexi (GP) comprise the intrinsic cardiac autonomic nervous system composed of autonomic ganglia of the heart atrium and ventricles. The GP are embedded in the epicardial fat pads, consisting of only a few neurons or as many as 400 neurons. GP are spatially close to the pulmonary veins, such that pulmonary vein isolation necessarily affects the GP. GP has been shown to be a contributor to atrial fibrillation (AFib), such that ablation of the GP has been a strategy for treatment of AFib. Addition of GP ablation to pulmonary vein isolation has not improved outcomes, but possibly other methods of GP ablation would be more successful. GP ablation alone has been shown to eliminate AFib in approximately three-quarter of AFib patients.
References
- ↑ Cox J, Schuessler R, D'Agostino H, Stone C, Chang B, Cain M, Corr P, Boineau J (1991). "The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure". J Thorac Cardiovasc Surg. 101 (4): 569–83. doi:10.1016/S0022-5223(19)36684-X. PMID 2008095.
- 1 2 Cox JL, Churyla A, McCarthy PM (2018). "When Is a Maze Procedure a Maze Procedure?". Canadian Journal of Cardiology . 34 (11): 1482–1491. doi:10.1016/j.cjca.2018.05.008. PMID 30121148.
- ↑ Wolf RK (2021). "Surgical Treatment of Atrial Fibrillation". Methodist DeBakey Cardiovascular Journal. 17 (1): 56–64. doi:10.14797/VNDG5944. PMC 5834122 . PMID 34104322.
- ↑ Prasad S, Maniar H, Camillo C, Schuessler R, Boineau J, Sundt T, Cox J, Damiano R (2003). "The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures". J Thorac Cardiovasc Surg. 126 (6): 1822–8. doi: 10.1016/S0022-5223(03)01287-X . PMID 14688693.
- ↑ Szalay Z, Skwara W, Pitschner H, Faude I, Klövekorn W, Bauer E (1999). "Midterm results after the mini-maze procedure". Eur J Cardiothorac Surg. 16 (3): 306–11. doi: 10.1016/S1010-7940(99)00208-0 . PMID 10554849.
- ↑ Cox J (2004). "The Role of Surgical Intervention in the Management of Atrial Fibrillation". Tex Heart Inst J. 31 (3): 257–65. PMC 521766 . PMID 15562846.
- 1 2 Saltman A, Rosenthal L, Francalancia N, Lahey S (2003). "A completely endoscopic approach to microwave ablation for atrial fibrillation". Heart Surg Forum. 6 (3): E38–41. PMID 12821436.
- 1 2 3 Salenger R, Lahey S, Saltman A (2004). "The completely endoscopic treatment of atrial fibrillation: report on the first 14 patients with early results". Heart Surg Forum. 7 (6): E555–8. doi:10.1532/HSF98.20041111. PMID 15769685.
- 1 2 3 Wolf R, Schneeberger E, Osterday R, Miller D, Merrill W, Flege J, Gillinov A (2005). "Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation". J Thorac Cardiovasc Surg. 130 (3): 797–802. doi: 10.1016/j.jtcvs.2005.03.041 . PMID 16153931.
- 1 2 Coumel P (1994). "Paroxysmal atrial fibrillation: a disorder of autonomic tone?". Eur Heart J. 15 Suppl A: 9–16. doi:10.1093/eurheartj/15.suppl_a.9. PMID 8070496.
- 1 2 Ninet J, Roques X, Seitelberger R, Deville C, Pomar J, Robin J, Jegaden O, Wellens F, Wolner E, Vedrinne C, Gottardi R, Orrit J, Billes M, Hoffmann D, Cox J, Champsaur G (2005). "Surgical ablation of atrial fibrillation with off-pump, epicardial, high-intensity focused ultrasound: results of a multicenter trial". J Thorac Cardiovasc Surg. 130 (3): 803–9. doi: 10.1016/j.jtcvs.2005.05.014 . PMID 16153932.
- ↑ Scherlag B, Po S (2006). "The intrinsic cardiac nervous system and atrial fibrillation". Current Opinion in Cardiology. 21 (1): 51–4. doi:10.1097/01.hco.0000198980.40390.e4. PMID 16355030. S2CID 34469546.
- ↑ Pappone C, Santinelli V, Manguso F, Vicedomini G, Gugliotta F, Augello G, Mazzone P, Tortoriello V, Landoni G, Zangrillo A, Lang C, Tomita T, Mesas C, Mastella E, Alfieri O (2004). "Pulmonary vein denervation enhances long-term benefit after circumferential ablation for paroxysmal atrial fibrillation". Circulation. 109 (3): 327–34. doi: 10.1161/01.CIR.0000112641.16340.C7 . PMID 14707026.
- ↑ Scherlag B, Nakagawa H, Jackman W, Yamanashi W, Patterson E, Po S, Lazzara R (2005). "Electrical stimulation to identify neural elements on the heart: their role in atrial fibrillation". J Interv Card Electrophysiol. 13 (Suppl 1): 37–42. doi:10.1007/s10840-005-2492-2. PMID 16133854. S2CID 19856445.
- ↑ Sunderland, N., Maruthappu, M. & Nagendran, M. (2011). "What size of left atrium significantly impairs the success of maze surgery for atrial fibrillation?". Interact Cardiovasc Thorac Surg. 13 (3): 332–8. doi: 10.1510/icvts.2011.271999 . PMID 21632865.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ↑ Krul SP, Driessen AH, de Groot JR (2013). "Navigating the mini-maze: systematic review of the first results and progress of minimally-invasive surgery in the treatment of atrial fibrillation". International Journal of Cardiology . 168 (1): 132–140. doi:10.1016/j.ijcard.2011.10.011. PMID 22078990.
- ↑ Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ (2007). "HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation". Heart Rhythm. 4 (6): 816–61. doi:10.1016/j.hrthm.2007.04.005. PMID 17556213.
- ↑ Israel C, Grönefeld G, Ehrlich J, Li Y, Hohnloser S (2004). "Long-term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care". J Am Coll Cardiol. 43 (1): 47–52. doi: 10.1016/j.jacc.2003.08.027 . PMID 14715182.
- ↑ McClelland JH, Duke D, Reddy R (2007). "Preliminary results of a limited thoracotomy: new approach to treat atrial fibrillation". J Cardiovasc Electrophysiol. 18 (12): 1296–8. doi:10.1111/j.1540-8167.2007.00977.x. PMID 17919294. S2CID 13944757.
- ↑ Wudel JH, Chaudhuri P, Hiller JJ (2008). "Video-assisted epicardial ablation and left atrial appendage exclusion for atrial fibrillation: extended follow-up". Ann Thorac Surg. 85 (1): 34–8. doi:10.1016/j.athoracsur.2007.08.014. PMID 18154774.