Related Research Articles
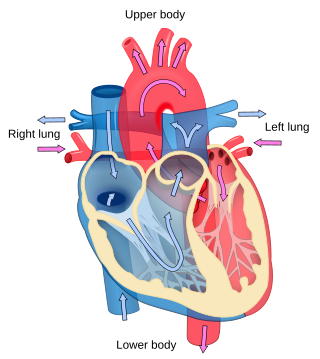
Cardiology is the study of the heart. Cardiology is a branch of medicine that deals with disorders of the heart and the cardiovascular system. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease, and electrophysiology. Physicians who specialize in this field of medicine are called cardiologists, a specialty of internal medicine. Pediatric cardiologists are pediatricians who specialize in cardiology. Physicians who specialize in cardiac surgery are called cardiothoracic surgeons or cardiac surgeons, a specialty of general surgery.
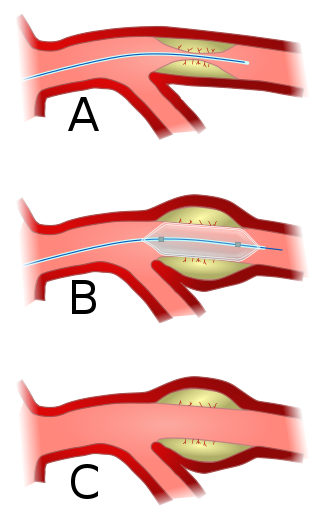
Angioplasty, also known as balloon angioplasty and percutaneous transluminal angioplasty (PTA), is a minimally invasive endovascular procedure used to widen narrowed or obstructed arteries or veins, typically to treat arterial atherosclerosis. A deflated balloon attached to a catheter is passed over a guide-wire into the narrowed vessel and then inflated to a fixed size. The balloon forces expansion of the blood vessel and the surrounding muscular wall, allowing an improved blood flow. A stent may be inserted at the time of ballooning to ensure the vessel remains open, and the balloon is then deflated and withdrawn. Angioplasty has come to include all manner of vascular interventions that are typically performed percutaneously.

Aortic stenosis is the narrowing of the exit of the left ventricle of the heart, such that problems result. It may occur at the aortic valve as well as above and below this level. It typically gets worse over time. Symptoms often come on gradually with a decreased ability to exercise often occurring first. If heart failure, loss of consciousness, or heart related chest pain occur due to AS the outcomes are worse. Loss of consciousness typically occurs with standing or exercising. Signs of heart failure include shortness of breath especially when lying down, at night, or with exercise, and swelling of the legs. Thickening of the valve without narrowing is known as aortic sclerosis.

The aortic valve is a valve in the heart of humans and most other animals, located between the left ventricle and the aorta. It is one of the four valves of the heart and one of the two semilunar valves, the other being the pulmonary valve. The aortic valve normally has three cusps or leaflets, although in 1–2% of the population it is found to congenitally have two leaflets. The aortic valve is the last structure in the heart the blood travels through before stopping the flow through the systemic circulation.

Tetralogy of Fallot (TOF), formerly known as Steno-Fallot tetralogy, is a congenital heart defect characterized by four specific cardiac defects. Classically, the four defects are:
Interventional cardiology is a branch of cardiology that deals specifically with the catheter based treatment of structural heart diseases. Andreas Gruentzig is considered the father of interventional cardiology after the development of angioplasty by interventional radiologist Charles Dotter.
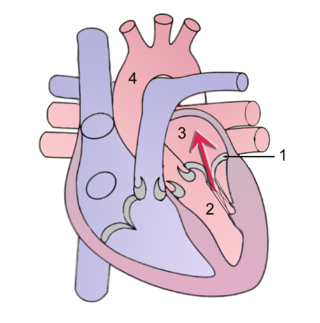
Mitral regurgitation(MR), also known as mitral insufficiency or mitral incompetence, is a form of valvular heart disease in which the mitral valve is insufficient and does not close properly when the heart pumps out blood. It is the abnormal leaking of blood backwards – regurgitation from the left ventricle, through the mitral valve, into the left atrium, when the left ventricle contracts. Mitral regurgitation is the most common form of valvular heart disease.
Aortic valve replacement is a procedure whereby the failing aortic valve of a patient's heart is replaced with an artificial heart valve. The aortic valve may need to be replaced because:

Cardiac catheterization is the insertion of a catheter into a chamber or vessel of the heart. This is done both for diagnostic and interventional purposes.
The Rastelli procedure is an open heart surgical procedure developed by Italian physician and cardiac surgery researcher, Giancarlo Rastelli, in 1967 at the Mayo Clinic, and involves using a pulmonary or aortic homograft conduit to relieve pulmonary obstruction in double outlet right ventricle with pulmonary stenosis.
Arterial switch operation (ASO) or arterial switch, is an open heart surgical procedure used to correct dextro-transposition of the great arteries (d-TGA).

A drug-eluting stent (DES) is a thin tube that is used to treat narrowed arteries in medical procedures. It releases drugs to prevent the growth of scar tissue and reduce the risk of stent restenosis, which is the narrowing of the stented area of an artery after treatment. A drug-eluting stent is different from other types of stents because it has a coating that delivers medication directly to the arterial wall. A DES is often made of metal alloys and can be inserted into blocked or narrowed arteries through a catheter placed in a peripheral artery, such as in the arm or leg. DES is fully integrated with a catheter delivery system and is viewed as one integrated medical device.

Transcatheter aortic valve replacement (TAVR) is the replacement of the aortic valve of the heart through the blood vessels. The replacement valve is delivered via one of several access methods: transfemoral, transapical, subclavian, direct aortic, and transcaval, among others.

The Ross procedure, also known as pulmonary autograft, is a heart valve replacement operation to treat severe aortic valve disease, such as in children and young adults with a bicuspid aortic valve. It involves removing the diseased aortic valve, situated at the exit of the left side of the heart, and replacing it with the person's own healthy pulmonary valve (autograft), removed from the exit of the heart's right side. To reconstruct the right sided exit, a pulmonary valve from a cadaver (homograft), or a stentless xenograft, is used to replace the removed pulmonary valve. Compared to a mechanical valve replacement, it avoids the requirement for thinning the blood, has favourable blood flow dynamics, allows growth of the valve with growth of the child and has less risk of endocarditis.
The following outline is provided as an overview of and topical guide to cardiology, the branch of medicine dealing with disorders of the human heart. The field includes medical diagnosis and treatment of congenital heart defects, coronary artery disease, heart failure, valvular heart disease and electrophysiology. Physicians who specialize in cardiology are called cardiologists.
Impella is a family of medical devices used for temporary ventricular support in patients with depressed heart function. Some versions of the device can provide left heart support during other forms of mechanical circulatory support including ECMO and Centrimag.

A hybrid cardiac surgical procedure in a narrow sense is defined as a procedure that combines a conventional, more invasive surgical part with an interventional part, using some sort of catheter-based procedure guided by fluoroscopy imaging in a hybrid operating room (OR) without interruption. The hybrid technique has a reduced risk of surgical complications and has shown decreased recovery time. It can be used to treat numerous heart diseases and conditions and with the increasing complexity of each case, the hybrid surgical technique is becoming more common.
Barry A. Love is an American cardiologist specializing in pediatric and congenital heart problems.
The LeCompte maneuver is a technique used in open heart surgery, primarily on infants and children. The maneuver entails cutting the main pulmonary artery and moving it anterior to the aorta before reattaching the pulmonary artery during the following reconstruction of the great vessels. It allows the surgeon to reconstruct the right ventricular outflow tract without needing to connect the proximal and distal sections with a graft. It also enables the surgeon to avoid compressing the coronary arteries and relieves compression of the bronchi in cases where the pulmonary artery is severely dilated or aneurysmal. If both pulmonary arteries are not mobilized adequately, they can become stretched, leading to pulmonic stenosis.
The Yasui procedure is a pediatric heart operation used to bypass the left ventricular outflow tract (LVOT) that combines the aortic repair of the Norwood procedure and a shunt similar to that used in the Rastelli procedure in a single operation. It is used to repair defects that result in the physiology of hypoplastic left heart syndrome even though both ventricles are functioning normally. These defects are common in DiGeorge syndrome and include interrupted aortic arch and LVOT obstruction (IAA/LVOTO); aortic atresia-severe stenosis with ventricular septal defect (AA/VSD); and aortic atresia with interrupted aortic arch and aortopulmonary window. This procedure allows the surgeon to keep the left ventricle connected to the systemic circulation while using the pulmonary valve as its outflow valve, by connecting them through the ventricular septal defect. The Yasui procedure includes a modified Damus–Kaye–Stansel procedure to connect the aortic and pulmonary roots, allowing the coronary arteries to remain perfused. It was first described in 1987.
References
- 1 2 3 4 5 6 7 Wilson W, Osten M, Benson L, Horlick E (January 2014). "Evolving trends in interventional cardiology: endovascular options for congenital disease in adults". The Canadian Journal of Cardiology. 30 (1): 75–86. doi: 10.1016/j.cjca.2013.11.006 . PMID 24365192.
- ↑ "Percutaneous pulmonary valve implantation for right ventricular outflow tract dysfunction". NICE. 23 January 2013. Retrieved 25 March 2019.
- ↑ Jalal Z, Thambo JB, Boudjemline Y (November 2014). "The future of transcatheter pulmonary valvulation". Archives of Cardiovascular Diseases. 107 (11): 635–42. doi:10.1016/j.acvd.2014.07.046. PMID 25241221.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 Ansari MM, Cardoso R, Garcia D, Sandhu S, Horlick E, Brinster D, et al. (November 2015). "Percutaneous Pulmonary Valve Implantation: Present Status and Evolving Future". Journal of the American College of Cardiology. 66 (20): 2246–2255. doi:10.1016/j.jacc.2015.09.055. PMID 26564602.
- 1 2 3 Virk SA, Liou K, Chandrakumar D, Gupta S, Cao C (December 2015). "Percutaneous pulmonary valve implantation: A systematic review of clinical outcomes". International Journal of Cardiology. 201: 487–9. doi:10.1016/j.ijcard.2015.08.119. PMID 26313872.
- 1 2 3 4 5 6 7 8 9 Qureshi AM, Prieto LR (June 2015). "Percutaneous pulmonary valve placement". Texas Heart Institute Journal. 42 (3): 195–201. doi:10.14503/THIJ-14-4276. PMC 4473610 . PMID 26175629.
- ↑ Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, et al. (October 2000). "Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction". Lancet. 356 (9239): 1403–5. doi:10.1016/S0140-6736(00)02844-0. PMID 11052583. S2CID 38642691.
- ↑ "FDA Approves Edwards Sapien 3 Transcatheter Heart Valve". Cardiac Interventions Today. Retrieved 21 December 2020.
- ↑ de Torres-Alba F, Kaleschke G, Baumgartner H (October 2018). "Impact of Percutaneous Pulmonary Valve Implantation on the Timing of Reintervention for Right Ventricular Outflow Tract Dysfunction". Revista Espanola de Cardiologia. 71 (10): 838–846. doi:10.1016/j.rec.2018.05.001. PMID 29859895. S2CID 44167038.