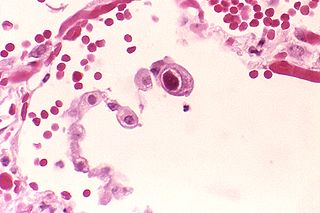
Cytomegalovirus (CMV) is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Betaherpesvirinae. Humans and other primates serve as natural hosts. The 11 species in this genus include human betaherpesvirus 5, which is the species that infects humans. Diseases associated with HHV-5 include mononucleosis and pneumonia, and congenital CMV in infants can lead to deafness and ambulatory problems.
Rhadinovirus is a genus of viruses in the order Herpesvirales, in the family Herpesviridae, in the subfamily Gammaherpesvirinae. Humans and other mammals serve as natural hosts. There are 12 species in this genus. Diseases associated with this genus include: Kaposi's sarcoma, primary effusion lymphoma and multicentric Castleman's disease, caused by Human gammaherpesvirus 8 (HHV-8), also known as Kaposi's sarcoma-associated herpesvirus (KSHV). The term rhadino comes from the Latin fragile, referring to the tendency of the viral genome to break apart when it is isolated.

Kaposi's sarcoma-associated herpesvirus (KSHV) is the ninth known human herpesvirus; its formal name according to the International Committee on Taxonomy of Viruses (ICTV) is Human gammaherpesvirus 8, or HHV-8 in short. Like other herpesviruses, its informal names are used interchangeably with its formal ICTV name. This virus causes Kaposi's sarcoma, a cancer commonly occurring in AIDS patients, as well as primary effusion lymphoma, HHV-8-associated multicentric Castleman's disease and KSHV inflammatory cytokine syndrome. It is one of seven currently known human cancer viruses, or oncoviruses. Even after many years since the discovery of KSHV/HHV8, there is no known cure for KSHV associated tumorigenesis.

Patrick S. Moore is an American virologist and epidemiologist who co-discovered together with his wife, Yuan Chang, two different human viruses causing the AIDS-related cancer Kaposi's sarcoma and the skin cancer Merkel cell carcinoma. Moore and Chang have discovered two of the seven known human viruses causing cancer. The couple met while in medical school together and were married in 1989 while they pursued fellowships at different universities.

Herpesviridae is a large family of DNA viruses that cause infections and certain diseases in animals, including humans. The members of this family are also known as herpesviruses. The family name is derived from the Greek word ἕρπειν, referring to spreading cutaneous lesions, usually involving blisters, seen in flares of herpes simplex 1, herpes simplex 2 and herpes zoster (shingles). In 1971, the International Committee on the Taxonomy of Viruses (ICTV) established Herpesvirus as a genus with 23 viruses among four groups. As of 2020, 115 species are recognized, all but one of which are in one of the three subfamilies. Herpesviruses can cause both latent and lytic infections.
The murine leukemia viruses are retroviruses named for their ability to cause cancer in murine (mouse) hosts. Some MLVs may infect other vertebrates. MLVs include both exogenous and endogenous viruses. Replicating MLVs have a positive sense, single-stranded RNA (ssRNA) genome that replicates through a DNA intermediate via the process of reverse transcription.

Gammaherpesvirinae is a subfamily of viruses in the order Herpesvirales and in the family Herpesviridae. Viruses in Gammaherpesvirinae are distinguished by reproducing at a more variable rate than other subfamilies of Herpesviridae. Mammals serve as natural hosts. There are 43 species in this subfamily, divided among 7 genera with three species unassigned to a genus. Diseases associated with this subfamily include: HHV-4: infectious mononucleosis. HHV-8: Kaposi's sarcoma.

Viral entry is the earliest stage of infection in the viral life cycle, as the virus comes into contact with the host cell and introduces viral material into the cell. The major steps involved in viral entry are shown below. Despite the variation among viruses, there are several shared generalities concerning viral entry.
Env is a viral gene that encodes the protein forming the viral envelope. The expression of the env gene enables retroviruses to target and attach to specific cell types, and to infiltrate the target cell membrane.
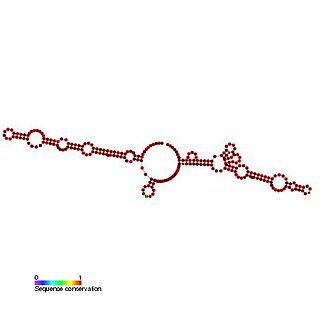
This family represents the Kaposi's sarcoma-associated herpesvirus (KSHV) internal ribosome entry site (IRES) present in the vCyclin gene. The vCyclin and vFLIP coding sequences are present on a bicistronic transcript and it is thought the IRES may initiate translation of vFLIP from this bicistronic transcript.

Poliovirus receptor-related 2 (PVRL2), also known as nectin-2 and CD112, is a human plasma membrane glycoprotein.

Poliovirus receptor-related 1 (PVRL1), also known as nectin-1 and CD111 (formerly herpesvirus entry mediator C, HVEC) is a human protein of the immunoglobulin superfamily (IgSF), also considered a member of the nectins. It is a membrane protein with three extracellular immunoglobulin domains, a single transmembrane helix and a cytoplasmic tail. The protein can mediate Ca2+-independent cellular adhesion further characterizing it as IgSF cell adhesion molecule (IgSF CAM).
Murid gammaherpesvirus 4 (MuHV-4) is a species of virus in the genus Rhadinovirus. It is a member of the subfamily Gammaherpesvirinae in the family Herpesviridae. This species infects mice via the nasal passages and causes an acute infectious mononucleosis-like syndrome with elevated levels of leukocytes, and shifts in the relative proportion of lymphocytes along with the appearance of atypical mononuclear cells. Murid gammaherpesvirus 4 currently serves as a model for study of human gammaherpesvirus pathogenesis.
The latency-associated nuclear antigen (LANA-1) or latent nuclear antigen is a Kaposi's sarcoma-associated herpesvirus (KSHV) latent protein initially found by Moore and colleagues as a speckled nuclear antigen present in primary effusion lymphoma cells that reacts with antibodies from patients with KS. It is the most immunodominant KSHV protein identified by Western-blotting as 222–234 kDa double bands migrate slower than the predicted molecular weight. LANA has been suspected of playing a crucial role in modulating viral and cellular gene expression. It is commonly used as an antigen in blood tests to detect antibodies in persons that have been exposed to KSHV.
Sarah A. Connolly is an American virologist. She graduated with a PhD from the University of Pennsylvania in 2003 and is notable for her work on Paramyxovirus and Herpes virus.

Herpesvirus glycoprotein B is a viral glycoprotein that is involved in the viral cell entry of Herpes simplex virus (HSV). Herpesviruses have a lipid bilayer, called the envelope, which contains twelve surface glycoproteins. For infectivity to be attained, the double stranded DNA genome of HSV must enter the host cell through means of fusion of its envelope with the cellular membrane or via endocytosis. Other viral glycoproteins involved in the process of viral cell entry include gC, gB, gD, gH, and gL, but only gC, gB, gD, and gH are required for the fusion of the HSV's envelope with the cellular membrane. It can be noted that all herpesviruses have glycoproteins gB, gH, and gL.
Eva Henriette Gottwein is a virologist and Associate Professor of Microbiology-Immunology at Northwestern University Feinberg School of Medicine in Chicago, Illinois. The main focus of her research is the role of viral miRNAs involved in herpesviral oncogenesis. Gottwein is member of Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Her contributions as a member include the focus on how encoded miRNAs target and function in the human oncogenic herpesvirus Kaposi's sarcoma-associated herpesvirus known as KSHV.
Human herpes viruses, also known as HHVs, are part of a family of DNA viruses that cause several diseases in humans. One of the most notable functions of this virus family is their ability to enter a latent phase and lay dormant within animals for extended periods of time. The mechanism that controls this is very complex because expression of viral proteins during latency is decreased a great deal, meaning that the virus must have transcription of its genes repressed. There are many factors and mechanisms that control this process and epigenetics is one way this is accomplished. Epigenetics refers to persistent changes in expression patterns that are not caused by changes to the DNA sequence. This happens through mechanisms such as methylation and acetylation of histones, DNA methylation, and non-coding RNAs (ncRNA). Altering the acetylation of histones creates changes in expression by changing the binding affinity of histones to DNA, making it harder or easier for transcription machinery to access the DNA. Methyl and acetyl groups can also act as binding sites for transcription factors and enzymes that further modify histones or alter the DNA itself.
HSV epigenetics is the epigenetic modification of herpes simplex virus (HSV) genetic code.
Ictavirus ictaluridallo1 (IcHV-1) is a species of virus in the genus Ictalurivirus, family Alloherpesviridae, and order Herpesvirales. It causes disease in channel catfish and blue catfish, and can cause significant economic loss in catfish farms. The disease is endemic in the USA and there are reports of the virus in Honduras and Russia.










