The Haber process, also called the Haber–Bosch process, is an artificial nitrogen fixation process and is the main industrial procedure for the production of ammonia today. It is named after its inventors, the German chemists Fritz Haber and Carl Bosch, who developed it in the first decade of the 20th century. The process converts atmospheric nitrogen (N2) to ammonia (NH3) by a reaction with hydrogen (H2) using a metal catalyst under high temperatures and pressures:

Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure, two atoms of the element bind to form N2, a colorless and odorless diatomic gas. N2 forms about 78% of Earth's atmosphere, making it the most abundant uncombined element. Nitrogen occurs in all organisms, primarily in amino acids (and thus proteins), in the nucleic acids (DNA and RNA) and in the energy transfer molecule adenosine triphosphate. The human body contains about 3% nitrogen by mass, the fourth most abundant element in the body after oxygen, carbon, and hydrogen. The nitrogen cycle describes movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere.

Pyridine is a basic heterocyclic organic compound with the chemical formula C5H5N. It is structurally related to benzene, with one methine group (=CH−) replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide.

Dinitrogen pentoxide is the chemical compound with the formula N2O5, also known as nitrogen pentoxide or nitric anhydride. It is one of the binary nitrogen oxides, a family of compounds that only contain nitrogen and oxygen. It exists as colourless crystals that melt at 41 °C. Its boiling point is 47 °C, and sublimes slightly above room temperature, yielding a colorless gas.
In chemistry, catenation is the bonding of atoms of the same element into a series, called a chain. A chain or a ring shape may be open if its ends are not bonded to each other, or closed if they are bonded in a ring. The words to catenate and catenation reflect the Latin root catena, "chain".
In chemistry, a nitrene or imene (R–N) is the nitrogen analogue of a carbene. The nitrogen atom is uncharged and univalent, so it has only 6 electrons in its valence level—two covalent bonded and four non-bonded electrons. It is therefore considered an electrophile due to the unsatisfied octet. A nitrene is a reactive intermediate and is involved in many chemical reactions. The simplest nitrene, HN, is called imidogen, and that term is sometimes used as a synonym for the nitrene class.

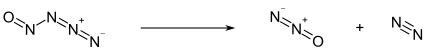
Hydrazoic acid, also known as hydrogen azide or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive liquid at room temperature and pressure. It is a compound of nitrogen and hydrogen, and is therefore a pnictogen hydride. It was first isolated in 1890 by Theodor Curtius. The acid has few applications, but its conjugate base, the azide ion, is useful in specialized processes.
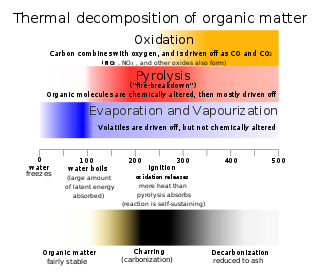
Thermal decomposition, or thermolysis, is a chemical decomposition caused by heat. The decomposition temperature of a substance is the temperature at which the substance chemically decomposes. The reaction is usually endothermic as heat is required to break chemical bonds in the compound undergoing decomposition. If decomposition is sufficiently exothermic, a positive feedback loop is created producing thermal runaway and possibly an explosion or other chemical reaction.
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring.

Nitroso refers to a functional group in organic chemistry which has the NO group attached to an organic moiety. As such, various nitroso groups can be categorized as C-nitroso compounds (e.g., nitrosoalkanes; R−N=O), S-nitroso compounds (nitrosothiols; RS−N=O), N-nitroso compounds (e.g., nitrosamines, R1N(−R2)−N=O), and O-nitroso compounds (alkyl nitrites; RO−N=O).

Uranium nitrides is any of a family of several ceramic materials: uranium mononitride (UN), uranium sesquinitride (U2N3) and uranium dinitride (UN2). The word nitride refers to the −3 oxidation state of the nitrogen bound to the uranium.
A nitrenium ion in organic chemistry is a reactive intermediate based on nitrogen with both an electron lone pair and a positive charge and with two substituents. Nitrenium ions are isoelectronic with carbenes, and can exist in either a singlet or a triplet state. The parent nitrenium ion, NH+2, is a ground state triplet species with a gap of 30 kcal/mol (130 kJ/mol) to the lowest energy singlet state. Conversely, most arylnitrenium ions are ground state singlets. Certain substituted arylnitrenium ions can be ground state triplets, however. Nitrenium ions can have microsecond or longer lifetimes in water.

Tetranitrogen is a neutrally charged polynitrogen allotrope of the chemical formula N
4 and consists of four nitrogen atoms. The tetranitrogen cation is the positively charged ion, N+
4, which is more stable than the neutral tetranitrogen molecule and is thus more studied.
The term bioorthogonal chemistry refers to any chemical reaction that can occur inside of living systems without interfering with native biochemical processes. The term was coined by Carolyn R. Bertozzi in 2003. Since its introduction, the concept of the bioorthogonal reaction has enabled the study of biomolecules such as glycans, proteins, and lipids in real time in living systems without cellular toxicity. A number of chemical ligation strategies have been developed that fulfill the requirements of bioorthogonality, including the 1,3-dipolar cycloaddition between azides and cyclooctynes, between nitrones and cyclooctynes, oxime/hydrazone formation from aldehydes and ketones, the tetrazine ligation, the isocyanide-based click reaction, and most recently, the quadricyclane ligation.

Cyanuric triazide (C3N12 or (NCN3)3) is described as an environmentally friendly, low toxicity, and organic primary explosive with a detonation velocity of about 7,300 m s−1, and ignition temperature at 205 °C. Primary research on this compound focuses on its use as a high energy density compound.
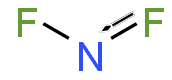
Nitrogen difluoride, also known as difluoroamino, is a reactive radical molecule with formula NF2. This small molecule is in equilibrium with its dimer dinitrogen tetrafluoride.
In chemistry, pyramidal inversion is a fluxional process in compounds with a pyramidal molecule, such as ammonia (NH3) "turns inside out". It is a rapid oscillation of the atom and substituents, the molecule or ion passing through a planar transition state. For a compound that would otherwise be chiral due to a stereocenter, pyramidal inversion allows its enantiomers to racemize.
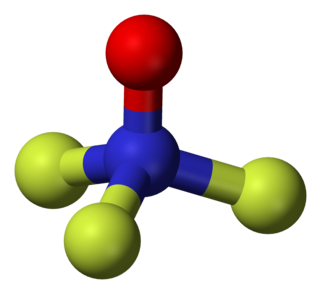
Trifluoramine oxide or Nitrogen trifluoride oxide (F3NO) is an inorganic molecule with strong fluorinating powers.

1-Diazidocarbamoyl-5-azidotetrazole, often jokingly referred to as azidoazide azide, is a heterocyclic inorganic compound with the formula C2N14. It is an extremely sensitive explosive.
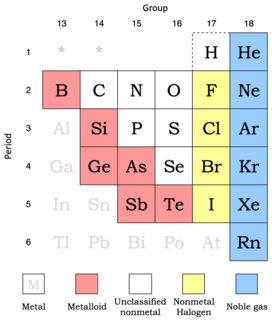
Nonmetals show more variability in their properties than do metals. Metalloids are included here since they behave predominately as chemically weak nonmetals.