The General Conference on Weights and Measures is the supreme authority of the International Bureau of Weights and Measures (BIPM), the intergovernmental organization established in 1875 under the terms of the Metre Convention through which member states act together on matters related to measurement science and measurement standards. The CGPM is made up of delegates of the governments of the member states and observers from the Associates of the CGPM. It elects the International Committee for Weights and Measures as the supervisory board of the BIPM to direct and supervise it.

The kilogram is the base unit of mass in the International System of Units (SI), having the unit symbol kg. 'Kilogram' means 'one thousand grams' and is colloquially abbreviated to kilo.
The katal is that catalytic activity that will raise the rate of conversion by one mole per second in a specified assay system. It is a unit of the International System of Units (SI) used for quantifying the catalytic activity of enzymes and other catalysts.

The litre or liter is a metric unit of volume. It is equal to 1 cubic decimetre (dm3), 1000 cubic centimetres (cm3) or 0.001 cubic metres (m3). A cubic decimetre occupies a volume of 10 cm × 10 cm × 10 cm and is thus equal to one-thousandth of a cubic metre.
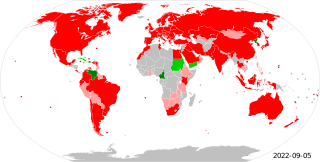
The Metre Convention, also known as the Treaty of the Metre, is an international treaty that was signed in Paris on 20 May 1875 by representatives of 17 nations: Argentina, Austria-Hungary, Belgium, Brazil, Denmark, France, Germany, Italy, Peru, Portugal, Russia, Spain, Sweden and Norway, Switzerland, Ottoman Empire, United States of America, and Venezuela.

The International System of Units, internationally known by the abbreviation SI, is the modern form of the metric system and the world's most widely used system of measurement. It is the only system of measurement with official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce. The SI system is coordinated by the International Bureau of Weights and Measures which is abbreviated BIPM from French: Bureau international des poids et mesures.

The Avogadro constant, commonly denoted NA or L, is an SI defining constant with an exact value of 6.02214076×1023 mol−1 (reciprocal moles). It is this defined number of constituent particles (usually molecules, atoms, ions, or ion pairs—in general, entities) per mole (SI unit) and used as a normalization factor in relating the amount of substance, n(X), in a sample of a substance X to the corresponding number of entities, N(X): n(X) = N(X)(1/NA), an aggregate of N(X) reciprocal Avogadro constants. By setting N(X) = 1, a reciprocal Avogadro constant is seen to be equal to one entity, which means that n(X) is more easily interpreted as an aggregate of N(X) entities. In the SI dimensional analysis of measurement units, the dimension of the Avogadro constant is the reciprocal of amount of substance, denoted N−1. The Avogadro number, sometimes denoted N0, is the numeric value of the Avogadro constant (i.e., without a unit), namely the dimensionless number 6.02214076×1023; the value chosen based on the number of atoms in 12 grams of carbon-12 in alignment with the historical definition of a mole. The constant is named after the Italian physicist and chemist Amedeo Avogadro (1776–1856).
The dalton or unified atomic mass unit is a unit of mass defined as 1/12 of the mass of an unbound neutral atom of carbon-12 in its nuclear and electronic ground state and at rest. It is a non-SI unit accepted for use with SI. The atomic mass constant, denoted mu, is defined identically, giving mu = 1/12m(12C) = 1 Da.
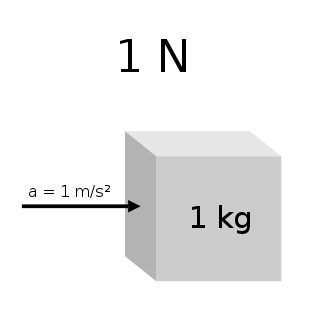
The newton is the unit of force in the International System of Units (SI). Expressed in terms of SI base units, it is 1 kg⋅m/s2, the force that accelerates a mass of one kilogram at one metre per second squared.

The gram is a unit of mass in the International System of Units (SI) equal to one thousandth of a kilogram.
The joule-second is the unit of action and of angular momentum in the International System of Units (SI) equal to the product of an SI derived unit, the joule (J), and an SI base unit, the second (s). The joule-second is a unit of action or of angular momentum. The joule-second also appears in quantum mechanics within the definition of the Planck constant. Angular momentum is the product of an object's moment of inertia, in units of kg⋅m2 and its angular velocity in units of rad⋅s−1. This product of moment of inertia and angular velocity yields kg⋅m2⋅s−1 or the joule-second. The Planck constant represents the energy of a wave, in units of joule, divided by the frequency of that wave, in units of s−1. This quotient of energy and frequency also yields the joule-second (J⋅s).
The vacuum magnetic permeability is the magnetic permeability in a classical vacuum. It is a physical constant, conventionally written as μ0. It quantifies the strength of the magnetic field induced by an electric current. Expressed in terms of SI base units, it has the unit kg⋅m⋅s−2⋅A−2. It can be also expressed in terms of SI derived units, N⋅A−2.
The molar mass constant, usually denoted by Mu, is a physical constant defined as one twelfth of the molar mass of carbon-12: Mu = M(12C)/12. The molar mass of an element or compound is its relative atomic mass or relative molecular mass multiplied by the molar mass constant.

The kelvin is the base unit for temperature in the International System of Units (SI). The Kelvin scale is an absolute temperature scale that starts at the lowest possible temperature, taken to be 0 K. By definition, the Celsius scale and the Kelvin scale have the exact same magnitude; that is, a rise of 1 K is equal to a rise of 1 °C and vice versa, and any temperature in degrees Celsius can be converted to kelvin by adding 273.15.
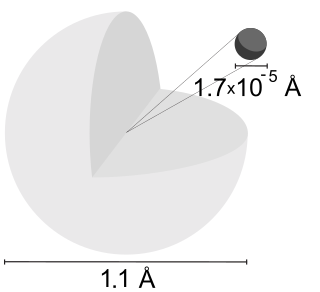
The angstrom is a unit of length equal to 10−10 m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–1874). It was originally spelled with Swedish letters, as Ångström and later as ångström. The latter spelling is still listed in some dictionaries, but is now rare in English texts. Some popular US dictionaries list only the spelling angstrom.

In metrology, a standard is an object, system, or experiment that bears a defined relationship to a unit of measurement of a physical quantity. Standards are the fundamental reference for a system of weights and measures, against which all other measuring devices are compared. Historical standards for length, volume, and mass were defined by many different authorities, which resulted in confusion and inaccuracy of measurements. Modern measurements are defined in relationship to internationally standardized reference objects, which are used under carefully controlled laboratory conditions to define the units of length, mass, electrical potential, and other physical quantities.

In 2019, four of the seven SI base units specified in the International System of Quantities were redefined in terms of natural physical constants, rather than human artefacts such as the standard kilogram. Effective 20 May 2019, the 144th anniversary of the Metre Convention, the kilogram, ampere, kelvin, and mole are now defined by setting exact numerical values, when expressed in SI units, for the Planck constant, the elementary electric charge, the Boltzmann constant, and the Avogadro constant, respectively. The second, metre, and candela had previously been redefined using physical constants. The four new definitions aimed to improve the SI without changing the value of any units, ensuring continuity with existing measurements. In November 2018, the 26th General Conference on Weights and Measures (CGPM) unanimously approved these changes, which the International Committee for Weights and Measures (CIPM) had proposed earlier that year after determining that previously agreed conditions for the change had been met. These conditions were satisfied by a series of experiments that measured the constants to high accuracy relative to the old SI definitions, and were the culmination of decades of research.

The history of the metric system began during the Age of Enlightenment with measures of length and weight derived from nature, along with their decimal multiples and fractions. The system became the standard of France and Europe within half a century. Other measures with unity ratios were added, and the system went on to be adopted across the world.
In physics, monochromatic radiation is electromagnetic radiation with a single constant frequency or wavelength. When that frequency is part of the visible spectrum the term monochromatic light is often used. Monochromatic light is perceived by the human eye as a spectral color.












