Related Research Articles

Maltase is one type of alpha-glucosidase enzymes located in the brush border of the small intestine. This enzyme catalyzes the hydrolysis of disaccharide maltose into two simple sugars of glucose. Maltase is found in plants, bacteria, yeast, humans, and other vertebrates. It is thought to be synthesized by cells of the mucous membrane lining the intestinal wall.
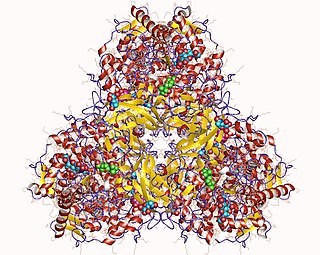
Glucan 1,4-α-glucosidase is an enzyme located on the brush border of the small intestine with systematic name 4-α-D-glucan glucohydrolase. It catalyses the following chemical reaction
The enzyme 1,5-anhydro-D-fructose dehydratase (EC 4.2.1.111) catalyzes the chemical reaction
The enzyme chondroitin AC lyase catalyzes the chemical reaction
The enzyme chondroitin B lyase catalyzes the following process:
The enzyme chondroitin-sulfate-ABC endolyase catalyzes the following process:
The enzyme chondroitin-sulfate-ABC exolyase catalyzes the following process:
The enzyme heparin lyase catalyzes the following process:
The enzyme hyaluronate lyase catalyzes the chemical reaction
Pectate lyase is an enzyme involved in the maceration and soft rotting of plant tissue. Pectate lyase is responsible for the eliminative cleavage of pectate, yielding oligosaccharides with 4-deoxy-α-D-mann-4-enuronosyl groups at their non-reducing ends. The protein is maximally expressed late in pollen development. It has been suggested that the pollen expression of pectate lyase genes might relate to a requirement for pectin degradation during pollen tube growth.
Pectin lyase, also known as pectolyase, is a naturally occurring pectinase, a type of enzyme that degrades pectin. It is produced commercially for the food industry from fungi and used to destroy residual fruit starch, known as pectin, in wine and cider. In plant cell culture, it is used in combination with the enzyme cellulase to generate protoplasts by degrading the plant cell walls.
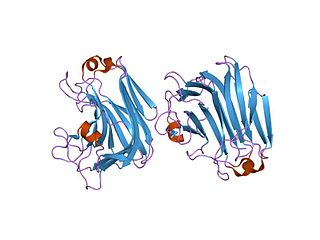
The enzyme mannuronate-specific alginate lyase catalyzes the degradation of alginate into various monosaccharide and polysaccharide products:
In enzymology, a polygalacturonate 4-alpha-galacturonosyltransferase is an enzyme that catalyzes the chemical reaction
PATE may refer to:

Endo-polygalacturonase (EC 3.2.1.15, pectin depolymerase, pectolase, pectin hydrolase, and poly-α-1,4-galacturonide glycanohydrolase; systematic name (1→4)-α-D-galacturonan glycanohydrolase (endo-cleaving)) is an enzyme that hydrolyzes the α-1,4 glycosidic bonds between galacturonic acid residues:
Chondroitin ABC lyase is an enzyme with systematic name chondroitin ABC lyase. This enzyme catalyses the following chemical reaction
The enzyme exo-(1→4)-α-D-glucan lyase (EC 4.2.2.13, α-(1→4)-glucan 1,5-anhydro-D-fructose eliminase, α-1,4-glucan exo-lyase, α-1,4-glucan lyase, GLase) is an enzyme with systematic name (1→4)-α-D-glucan exo-4-lyase (1,5-anhydro-D-fructose-forming). This enzyme catalyses the following chemical reaction
Pectate trisaccharide-lyase is an enzyme with systematic name (1→4)-α-D-galacturonan reducing-end-trisaccharide-lyase. This enzyme catalyses the following chemical reaction:
The enzyme Rhamnogalacturonan endolyase is an enzyme with systematic name α-L-rhamnopyranosyl-(1→4)-α-D-galactopyranosyluronate endolyase. catalyses the following process:
References
- Macmillan JD, Vaughn RH (April 1964). "Purification and properties of a polygalacturonic acid-trans-eliminase produced by Clostridium multifermentans". Biochemistry. 3 (4): 564–72. doi:10.1021/bi00892a016. PMID 14188174.