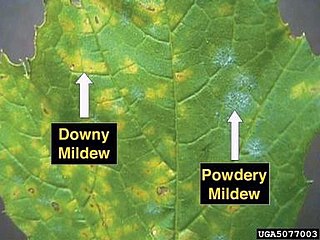
Downy mildew refers to any of several types of oomycete microbes that are obligate parasites of plants. Downy mildews exclusively belong to the Peronosporaceae family. In commercial agriculture, they are a particular problem for growers of crucifers, grapes and vegetables that grow on vines. The prime example is Peronospora farinosa featured in NCBI-Taxonomy and HYP3. This pathogen does not produce survival structures in the northern states of the United States, and overwinters as live mildew colonies in Gulf Coast states. It progresses northward with cucurbit production each spring. Yield loss associated with downy mildew is most likely related to soft rots that occur after plant canopies collapse and sunburn occurs on fruit. Cucurbit downy mildew only affects leaves of cucurbit plants.

Peronosporaceae are a family of water moulds that contains 21 genera, comprising more than 600 species. Most of them are called downy mildews.
Aphanomyces euteiches is a water mould, or oomycete, plant pathogen responsible for the disease Aphanomyces root rot. The species Aphanomyces euteiches can infect a variety of legumes. Symptoms of the disease can differ among hosts but generally include reduced root volume and function, leading to stunting and chlorotic foliage. Aphanomyces root rot is an important agricultural disease in the United States, Europe, Australia, New Zealand, and Japan. Management includes using resistant crop varieties and having good soil drainage, as well as testing soil for the pathogen to avoid infected fields.

Phytophthora medicaginis is an oomycete plant pathogen that causes root rot in alfalfa and chickpea. It is a major disease of these plants and is found wherever they are grown. P. medicaginis causes failure of stand establishment because of seedling death. Phytophthora medicaginis is part of a species complex with Phytophthora megasperma.
Pythium irregulare is a soil borne oomycete plant pathogen. Oomycetes, also known as "water molds", are fungal-like protists. They are fungal-like because of their similar life cycles, but differ in that the resting stage is diploid, they have coenocytic hyphae, a larger genome, cellulose in their cell walls instead of chitin, and contain zoospores and oospores.
Pythium ultimum is a plant pathogen. It causes damping off and root rot diseases of hundreds of diverse plant hosts including corn, soybean, potato, wheat, fir, and many ornamental species. P. ultimum belongs to the peronosporalean lineage of oomycetes, along with other important plant pathogens such as Phytophthora spp. and many genera of downy mildews. P. ultimum is a frequent inhabitant of fields, freshwater ponds, and decomposing vegetation in most areas of the world. Contributing to the widespread distribution and persistence of P. ultimum is its ability to grow saprotrophically in soil and plant residue. This trait is also exhibited by most Pythium spp. but not by the related Phytophthora spp., which can only colonize living plant hosts.

Phytophthora erythroseptica—also known as pink rot along with several other species of Phytophthora—is a plant pathogen. It infects potatoes causing their tubers to turn pink and damages leaves. It also infects tulips (Tulipa) damaging their leaves and shoots.
Sclerophthora macrospora is a protist plant pathogen of the class Oomycota. It causes downy mildew on a vast number of cereal crops including oats, rice, maize, and wheat as well as varieties of turf grass. The common names of the diseases associated with Sclerophthora macrospora include "crazy top disease" on maize and yellow tuft disease on turf grass. The disease is present all over the world, but it is especially persistent in Europe.

Diaporthe helianthi is a fungal pathogen that causes Phomopsis stem canker of sunflowers. In sunflowers, Phomopsis helianthi is the causative agent behind stem canker. Its primary symptom is the production of large canker lesions on the stems of sunflower plants. These lesions can eventually lead to lodging and plant death. This disease has been shown to be particularly devastating in southern and eastern regions of Europe, although it can also be found in the United States and Australia. While cultural control practices are the primary method of controlling for Stem Canker, there have been a few resistant cultivars developed in regions of Europe where the disease is most severe.
Aphanomyces cochlioides is a plant pathogen that can affect commodity crops like spinach, Swiss chard, beets and related species. In spinach the pathogen is responsible for the black root "rot" that can damage plants.

Bremia lactucae is a plant pathogen. This microorganism causes a disease of lettuce denominated as downy mildew. Some other strains can be found on 36 genera of Asteraceae including Senecio and Sonchus. Experiments using sporangia from hosts do not infect lettuce and it is concluded that the fungus exists as a quantity of host-specific strains. Wild species, such as Lactuca serriola, or varieties of Lactuca can hold strains that infect lettuce, but these pathogens are not sufficiently common to seriously infect the plant.

Peronospora manshurica is a plant pathogen. It is a widespread disease on the leaves of soybeans and other crop plants. The fungi is commonly referred to as downy mildew, "leafspot", or "leaf-spot".

Plasmopara viticola, the causal agent of grapevine downy mildew, is a heterothallic oomycete that overwinters as oospores in leaf litter and soil. In the spring, oospores germinate to produce macrosporangia, which under wet condition release zoospores. Zoospores are splashed by rain into the canopy, where they swim to and infect through stomata. After 7–10 days, yellow lesions appear on foliage. During favorable weather the lesions sporulate and new secondary infections occur.

Pseudoperonospora humuli is a plant pathogen that causes downy mildew on hops.
Phytophthora megakarya is an oomycete plant pathogen that causes black pod disease in cocoa trees in west and central Africa. This pathogen can cause detrimental loss of yield in the economically important cocoa industry, worth approximately $70 billion annually. It can damage any part of the tree, causing total yield losses which can easily reach 20-25%. A mixture of chemical and cultural controls, as well as choosing resistant plant varieties, are often necessary to control this pathogen.
Peronosclerospora sorghi is a plant pathogen. It is the causal agent of sorghum downy mildew. The pathogen is a fungal-like protist in the oomycota, or water mold, class. Peronosclerospora sorghi infects susceptible plants though sexual oospores, which survive in the soil, and asexual sporangia which are disseminated by wind. Symptoms of sorghum downy mildew include chlorosis, shredding of leaves, and death. Peronosclerospora sorghi infects maize and sorghum around the world, but causes the most severe yield reductions in Africa. The disease is controlled mainly through genetic resistance, chemical control, crop rotation, and strategic timing of planting.
Alternaria helianthi is a fungal plant pathogen causing a disease in sunflowers known as Alternaria blight of sunflower.

Pseudoperonospora cubensis is a species of water mould known for causing downy mildew on cucurbits such as cantaloupe, cucumber, pumpkin, squash and watermelon. This water mould is an important pathogen of all these crops, especially in areas with high humidity and rainfall, such as the eastern United States. In most years the disease is an annual, late-season problem on squash and pumpkin in the eastern and central United States, however, since 2004 it has become one of the most important diseases in cucumber production. Considered a highly destructive foliar disease of cucurbits, successful breeding in the mid-twentieth century provided adequate control of downy mildew in cucumber without the use of fungicides. The resurgence in virulence has caused growers great concern and substantial economic losses, while downy mildew in other cucurbit crops continues to be a yearly hindrance.

Peronospora destructor is a plant pathogen. It causes downy mildew on leaves of cultivated and wild Allium. Allium cepa is most often affected, while Allium schoenoprasum (chives) and Allium porrum (leek) are only occasionally affected.











