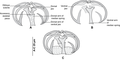
Microcotyle constricta is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.
Microcotyle longirostri is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.

Microcotyle donavini is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.
Microcotyle nemadactylus is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.
Microcotyle neozealanica is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.

Microcotyle elegans is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.
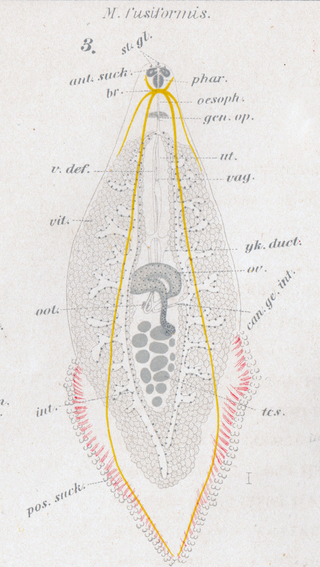
Microcotyle fusiformis is a species of monogenean, parasitic on the gills of a marine fish, described by Seitarō Gotō in 1894. It belongs to the family Microcotylidae. This species was first.
Microcotyle gimpo is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae.
Microcotyle sebastisci is a species of monogenean, parasitic on the gills of marine fish. It belongs to the family Microcotylidae.
Microcotyle pontica is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae. It was first described and illustrated from the gills of the east Atlantic peacock wrasse Symphodus tinca (Labridae), from the Black Sea.
Microcotyle inglisi is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae. It was first described and illustrated based on 5 specimens from the gills of the Indian mackerel Scomber microlepidotus (Scombridae) off Odisha, India..
Microcotyle korathai is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Microcotylidae. It was first described and illustrated based on 6 specimens from the gills of the Indian mackerel Scomber microlepidotus (Scombridae) off Odisha, India..
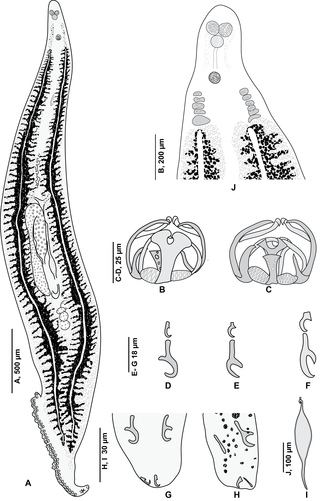
Pseudaxine is a genus which belongs to the phylum Platyhelminthes and class Monogenea; all its species are parasites of fish.
Pseudaxine indicana is a species of monogenean flatworm, which is parasitic on the gills of a marine fish. It belongs to the family Gastrocotylidae.
Pseudaxine kurra is a species of monogenean, parasitic on the gills of a marine fish. It belongs to the family Gastrocotylidae.

Pseudaxine bivaginalis is a species of monogenean flatworm, which is parasitic on the gills of a marine fish. It belongs to the family Gastrocotylidae.

Sibitrema is a genus which belongs to the phylum Platyhelminthes and class Monogenea; the only species included in this genus is parasite of fish.

Sibitrema poonui is a species of monogenean flatworm, which is parasitic on the gills of a marine fish. It belongs to the family Gastrocotylidae.

Allogastrocotyle bivaginalis is a species of monogenean flatworm, which is parasitic on the gills of a marine fish. It belongs to the family Gastrocotylidae.

Allopseudaxine yaito is a species of monogenean flatworm, which is parasitic on the gills of a marine fish. It belongs to the family Axinidae.