Related Research Articles

Heart murmurs are unique heart sounds produced when blood flows across a heart valve or blood vessel. This occurs when turbulent blood flow creates a sound loud enough to hear with a stethoscope. The sound differs from normal heart sounds by their characteristics. For example, heart murmurs may have a distinct pitch, duration and timing. The major way health care providers examine the heart on physical exam is heart auscultation; another clinical technique is palpation, which can detect by touch when such turbulence causes the vibrations called cardiac thrill. A murmur is a sign found during the cardiac exam. Murmurs are of various types and are important in the detection of cardiac and valvular pathologies.

A ventricle is one of two large chambers located toward the bottom of the heart that collect and expel blood towards the peripheral beds within the body and lungs. The blood pumped by a ventricle is supplied by an atrium, an adjacent chamber in the upper heart that is smaller than a ventricle. Interventricular means between the ventricles, while intraventricular means within one ventricle.
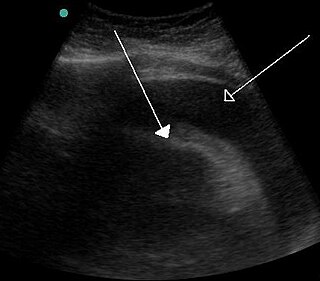
Cardiac tamponade, also known as pericardial tamponade, is a compression of the heart due to pericardial effusion. Onset may be rapid or gradual. Symptoms typically include those of obstructive shock including shortness of breath, weakness, lightheadedness, and cough. Other symptoms may relate to the underlying cause.
In cardiovascular physiology, stroke volume (SV) is the volume of blood pumped from the ventricle per beat. Stroke volume is calculated using measurements of ventricle volumes from an echocardiogram and subtracting the volume of the blood in the ventricle at the end of a beat from the volume of blood just prior to the beat. The term stroke volume can apply to each of the two ventricles of the heart, although when not explicitly stated it refers to the left ventricle and should therefore be referred to as left stroke volume (LSV). The stroke volumes for each ventricle are generally equal, both being approximately 90 mL in a healthy 70-kg man. Any persistent difference between the two stroke volumes, no matter how small, would inevitably lead to venous congestion of either the systemic or the pulmonary circulation, with a corresponding state of hypotension in the other circulatory system. A shunt between the two systems will ensue if possible to reestablish the equilibrium.

Afterload is the pressure that the heart must work against to eject blood during systole. Afterload is proportional to the average arterial pressure. As aortic and pulmonary pressures increase, the afterload increases on the left and right ventricles respectively. Afterload changes to adapt to the continually changing demands on an animal's cardiovascular system. Afterload is proportional to mean systolic blood pressure and is measured in millimeters of mercury.

Diastole is the relaxed phase of the cardiac cycle when the chambers of the heart are refilling with blood. The contrasting phase is systole when the heart chambers are contracting. Atrial diastole is the relaxing of the atria, and ventricular diastole the relaxing of the ventricles.

Mitral stenosis is a valvular heart disease characterized by the narrowing of the opening of the mitral valve of the heart. It is almost always caused by rheumatic valvular heart disease. Normally, the mitral valve is about 5 cm2 during diastole. Any decrease in area below 2 cm2 causes mitral stenosis. Early diagnosis of mitral stenosis in pregnancy is very important as the heart cannot tolerate increased cardiac output demand as in the case of exercise and pregnancy. Atrial fibrillation is a common complication of resulting left atrial enlargement, which can lead to systemic thromboembolic complications such as stroke.
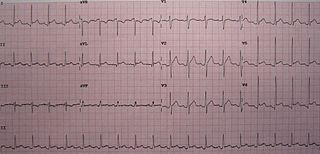
Pericarditis is inflammation of the pericardium, the fibrous sac surrounding the heart. Symptoms typically include sudden onset of sharp chest pain, which may also be felt in the shoulders, neck, or back. The pain is typically less severe when sitting up and more severe when lying down or breathing deeply. Other symptoms of pericarditis can include fever, weakness, palpitations, and shortness of breath. The onset of symptoms can occasionally be gradual rather than sudden.
Kussmaul's sign is a paradoxical rise in jugular venous pressure (JVP) on inspiration, or a failure in the appropriate fall of the JVP with inspiration. It can be seen in some forms of heart disease and is usually indicative of limited right ventricular filling due to right heart dysfunction.

The jugular venous pressure is the indirectly observed pressure over the venous system via visualization of the internal jugular vein. It can be useful in the differentiation of different forms of heart and lung disease. Classically three upward deflections and two downward deflections have been described.

A pulmonary artery catheter (PAC), also known as a Swan-Ganz catheter or right heart catheter, is a balloon-tipped catheter that is inserted into a pulmonary artery in a procedure known as pulmonary artery catheterization or right heart catheterization. Pulmonary artery catheterization is a useful measure of the overall function of the heart particularly in those with complications from heart failure, heart attack, arrhythmias or pulmonary embolism. It is also a good measure for those needing intravenous fluid therapy, for instance post heart surgery, shock, and severe burns. The procedure can also be used to measure pressures in the heart chambers.

In cardiac physiology, preload is the amount of sarcomere stretch experienced by cardiac muscle cells, called cardiomyocytes, at the end of ventricular filling during diastole. Preload is directly related to ventricular filling. As the relaxed ventricle fills during diastole, the walls are stretched and the length of sarcomeres increases. Sarcomere length can be approximated by the volume of the ventricle because each shape has a conserved surface-area-to-volume ratio. This is useful clinically because measuring the sarcomere length is destructive to heart tissue. It requires cutting out a piece of cardiac muscle to look at the sarcomeres under a microscope. It is currently not possible to directly measure preload in the beating heart of a living animal. Preload is estimated from end-diastolic ventricular pressure and is measured in millimeters of mercury (mmHg).
Venous return is the rate of blood flow back to the heart. It normally limits cardiac output.
Obstructive shock is one of the four types of shock, caused by a physical obstruction in the flow of blood. Obstruction can occur at the level of the great vessels or the heart itself. Causes include pulmonary embolism, cardiac tamponade, and tension pneumothorax. These are all life-threatening. Symptoms may include shortness of breath, weakness, or altered mental status. Low blood pressure and tachycardia are often seen in shock. Other symptoms depend on the underlying cause.

A split S2 is a finding upon auscultation of the S2 heart sound.
The E/A ratio is a marker of the function of the left ventricle of the heart. It represents the ratio of peak velocity blood flow from left ventricular relaxation in early diastole to peak velocity flow in late diastole caused by atrial contraction. It is calculated using Doppler echocardiography, an ultrasound-based cardiac imaging modality. Abnormalities in the E/A ratio suggest that the left ventricle, which pumps blood into the systemic circulation, cannot fill with blood properly in the period between contractions. This phenomenon is referred to as diastolic dysfunction and can eventually lead to the symptoms of heart failure.
Cardiac physiology or heart function is the study of healthy, unimpaired function of the heart: involving blood flow; myocardium structure; the electrical conduction system of the heart; the cardiac cycle and cardiac output and how these interact and depend on one another.
A plot of a system's pressure versus volume has long been used to measure the work done by the system and its efficiency. This analysis can be applied to heat engines and pumps, including the heart. A considerable amount of information on cardiac performance can be determined from the pressure vs. volume plot. A number of methods have been determined for measuring PV-loop values experimentally.

Heart failure with preserved ejection fraction (HFpEF) is a form of heart failure in which the ejection fraction – the percentage of the volume of blood ejected from the left ventricle with each heartbeat divided by the volume of blood when the left ventricle is maximally filled – is normal, defined as greater than 50%; this may be measured by echocardiography or cardiac catheterization. Approximately half of people with heart failure have preserved ejection fraction, while the other half have a reduction in ejection fraction, called heart failure with reduced ejection fraction (HFrEF).
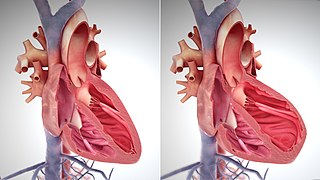
The main pathophysiology of heart failure is a reduction in the efficiency of the heart muscle, through damage or overloading. As such, it can be caused by a wide number of conditions, including myocardial infarction, hypertension and cardiac amyloidosis. Over time these increases in workload will produce changes to the heart itself:
References
- 1 2 3 4 Khasnis, A.; Lokhandwala, Y. (January–March 2002). "Clinical signs in medicine: pulsus paradoxus". Journal of Postgraduate Medicine. 48 (1). Mumbai - 400 012, India: 49: 46–9. ISSN 0022-3859. PMID 12082330 . Retrieved 21 March 2010.
The "paradox" refers to the fact that heart sounds may be heard over the precordium when the radial pulse is not felt.
{{cite journal}}: CS1 maint: location (link) - ↑ Guntheroth W, Morgan B, Mullins G (1967). "Effect of respiration on venous return and stroke volume in cardiac tamponade. Mechanism of pulsus parodoxus". Circ. Res. 20 (4): 381–90. doi: 10.1161/01.res.20.4.381 . PMID 6025402. Abstract Archived 1 February 2009 at the Wayback Machine
- ↑ Soucek M, Kára T, Jurák P, Halámek J, Spinarová L, Meluzín J, Toman J, Rihácek I, Sumbera J, Frána P (2003). "Heart rate and increased intravascular volume". Physiological Research. 52 (1): 137–40. doi: 10.33549/physiolres.930259 . PMID 12625819. Free Full Text.
- ↑ Van Dam M, Fitzgerald B (2024). "Pulsus Paradoxus". National Center for Biotechnology Information, U.S. National Library of Medicine. PMID 29493917 . Retrieved 11 July 2021.
- ↑ Van Dam M, Fitzgerald B (2024). "Pulsus Paradoxus". National Center for Biotechnology Information, U.S. National Library of Medicine. PMID 29493917 . Retrieved 11 July 2021.
- ↑ Reddy, PS; Curtiss, EI; Uretsky, BF (1990). "Spectrum of hemodynamic changes in cardiac tamponade". American Journal of Cardiology. 66 (20): 1487–91. doi:10.1016/0002-9149(90)90540-H. PMID 2251997.
- ↑ Reddy, PS; Curtiss, EI; O'Toole, JD; Shaver, JA (1978). "Cardiac tamponade: hemodynamic observations in man". Circulation. 58 (2): 265–72. doi: 10.1161/01.cir.58.2.265 . PMID 668074.
- ↑ Van Dam M, Fitzgerald B (2024). "Pulsus Paradoxus". National Center for Biotechnology Information, U.S. National Library of Medicine. PMID 29493917 . Retrieved 11 July 2021.
- ↑ Van Dam M, Fitzgerald B (2024). "Pulsus Paradoxus". National Center for Biotechnology Information, U.S. National Library of Medicine. PMID 29493917 . Retrieved 11 July 2021.
- ↑ Van Dam M, Fitzgerald B (2024). "Pulsus Paradoxus". National Center for Biotechnology Information, U.S. National Library of Medicine. PMID 29493917 . Retrieved 11 July 2021.
- ↑ Talreja, DR; Nishimura, RA; Oh, JK; Holmes, DR (22 January 2008). "Constrictive pericarditis in the modern era: novel criteria for diagnosis in the cardiac catheterization laboratory". Journal of the American College of Cardiology. 51 (3): 315–9. doi: 10.1016/j.jacc.2007.09.039 . PMID 18206742.
- ↑ Curtiss EI, Reddy PS, Uretsky BF, Cecchetti AA (February 1988). "Pulsus paradoxus: definition and relation to the severity of cardiac tamponade". Am Heart J. 115 (2): 391–8. doi:10.1016/0002-8703(88)90487-5. PMID 3341174.
{{cite journal}}: CS1 maint: multiple names: authors list (link)