
An infection is the invasion of an organism's body tissues by disease-causing agents, their multiplication, and the reaction of host tissues to the infectious agents and the toxins they produce. An infectious disease, also known as a transmissible disease or communicable disease, is an illness resulting from an infection.

An epidemic is the rapid spread of disease to a large number of people in a given population within a short period of time. For example, in meningococcal infections, an attack rate in excess of 15 cases per 100,000 people for two consecutive weeks is considered an epidemic.
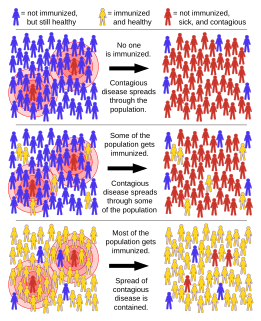
Herd immunity is a form of indirect protection from infectious disease that occurs when a sufficient percentage of a population has become immune to an infection, whether through vaccination or previous infections, thereby reducing the likelihood of infection for individuals who lack immunity. Immune individuals are unlikely to contribute to disease transmission, disrupting chains of infection, which stops or slows the spread of disease. The greater the proportion of immune individuals in a community, the smaller the probability that non-immune individuals will come into contact with an infectious individual.

Severe acute respiratory syndrome (SARS) is a viral respiratory disease of zoonotic origin caused by severe acute respiratory syndrome coronavirus, the first-identified strain of the SARS coronavirus species severe acute respiratory syndrome-related coronavirus (SARSr-CoV). The syndrome caused the 2002–2004 SARS outbreak. In late 2017, Chinese scientists traced the virus through the intermediary of Asian palm civets to cave-dwelling horseshoe bats in Yunnan.

An asymptomatic carrier is a person or other organism that has become infected with a pathogen, but that displays no signs or symptoms.

In medicine, public health, and biology, transmission is the passing of a pathogen causing communicable disease from an infected host individual or group to a particular individual or group, regardless of whether the other individual was previously infected.

In epidemiology, the basic reproduction number, or basic reproductive number, denoted , of an infection can be thought of as the expected number of cases directly generated by one case in a population where all individuals are susceptible to infection. The definition describes the state where no other individuals are infected or immunized. Some definitions, such as that of the Australian Department of Health, add absence of "any deliberate intervention in disease transmission". The basic reproduction number is not to be confused with the effective reproduction number , which is the number of cases generated in the current state of a population, which does not have to be the uninfected state. Also, it is important to note that is a dimensionless number and not a rate, which would have units of time−1, or units of time like doubling time.
Mathematical models can project how infectious diseases progress to show the likely outcome of an epidemic and help inform public health interventions. Models use basic assumptions or collected statistics along with mathematics to find parameters for various infectious diseases and use those parameters to calculate the effects of different interventions, like mass vaccination programmes. The modelling can help decide which intervention/s to avoid and which to trial, or can predict future growth patterns, etc.

In public health, contact tracing is the process of identification of persons who may have come into contact with an infected person ("contacts") and subsequent collection of further information about these contacts. By tracing the contacts of infected individuals, testing them for infection, isolating or treating the infected and tracing their contacts in turn, public health aims to reduce infections in the population. Diseases for which contact tracing is commonly performed include tuberculosis, vaccine-preventable infections like measles, sexually transmitted infections, blood-borne infections, Ebola, some serious bacterial infections, and novel virus infections. The goals of contact tracing are:

Meningococcal disease describes infections caused by the bacterium Neisseria meningitidis. It has a high mortality rate if untreated but is vaccine-preventable. While best known as a cause of meningitis, it can also result in sepsis, which is an even more damaging and dangerous condition. Meningitis and meningococcemia are major causes of illness, death, and disability in both developed and under-developed countries.
An emergent virus is a virus that is either newly appeared, notably increasing in incidence/geographic range or has the potential to increase in the near future. Emergent viruses are a leading cause of emerging infectious diseases and raise public health challenges globally, given their potential to cause outbreaks of disease which can lead to epidemics and pandemics. As well as causing disease, emergent viruses can also have severe economic implications. Recent examples include the SARS-related coronaviruses, which have caused the 2002-2004 outbreak of SARS (SARS-CoV-1) and the 2019–20 pandemic of COVID-19 (SARS-CoV-2). Other examples include the human immunodeficiency virus which causes HIV/AIDS; the viruses responsible for Ebola; the H5N1 influenza virus responsible for avian flu; and H1N1/09, which caused the 2009 swine flu pandemic. Viral emergence in humans is often a consequence of zoonosis, which involves a cross-species jump of a viral disease into humans from other animals. As zoonotic viruses exist in animal reservoirs, they are much more difficult to eradicate and can therefore establish persistent infections in human populations.
The index case or patient zero is the first documented patient in a disease epidemic within a population, or the first documented patient included in an epidemiological study. It can also refer to the first case of a condition or syndrome to be described in the medical literature, whether or not the patient is thought to be the first person affected. An index case can achieve the status of a "classic" case study in the literature, as did Phineas Gage, the first known person to exhibit a definitive personality change as a result of a brain injury.

Antibody-dependent enhancement (ADE), sometimes less precisely called immune enhancement or disease enhancement, is a phenomenon in which binding of a virus to suboptimal antibodies enhances its entry into host cells, followed by its replication. Antiviral antibodies promote viral infection of target immune cells by exploiting the phagocytic FcγR or complement pathway. After interaction with the virus the antibody binds Fc receptors (FcR) expressed on certain immune cells or some of the complement proteins. FcγR binds antibody via its fragment crystallizable region (Fc). This interaction facilitates phagocytosis of the virus-antibody complex by the immune cells. Usually this phagocytosis is accompanied by the virus degradation, but in the case of ADE, it can, on the contrary, cause viral replication, with the subsequent death of immune cells. Thus, the virus “deceives” the process of phagocytosis of immune cells and uses the host's antibodies as a “Trojan horse”. ADE can be induced when the strength of antibody-antigen interaction is below the certain threshold. This phenomenon might lead to both increased virus infectivity and virulence. The viruses that can cause ADE frequently share some common features such as antigenic diversity, abilities to replicate and establish persistence in immune cells. ADE can occur during the development of a primary or secondary viral infection, as well as after vaccination with a subsequent virus challenge. It has been observed mainly with positive-strand RNA viruses. Among them are Flaviviruses such as Dengue virus, Yellow fever virus, Zika virus, Coronaviruses, including alpha- and betacoronaviruses, Orthomyxoviruses such as influenza, Retroviruses such as HIV, and Orthopneumoviruses such as RSV.

Sunetra Gupta is an Indian infectious disease epidemiologist and a professor of theoretical epidemiology at the Department of Zoology, University of Oxford. She has performed research on the transmission dynamics of various infectious diseases, including malaria, influenza and COVID-19, and has received the Scientific Medal of the Zoological Society of London and the Rosalind Franklin Award of the Royal Society. Gupta is also a novelist and a recipient of the Sahitya Akademi Award.

Wildlife trafficking practices have resulted in the emergence of zoonotic diseases. Exotic wildlife trafficking is a multi-billion dollar industry that involves the removal and shipment of mammals, reptiles, amphibians, invertebrates, and fish all over the world. Traded wild animals are used for bushmeat consumption, unconventional exotic pets, animal skin clothing accessories, home trophy decorations, privately owned zoos, and for traditional medicine practices. Dating back centuries, people from Africa, Asia, Latin America, the Middle East, and Europe have used animal bones, horns, or organs for their believed healing effects on the human body. Wild tigers, rhinos, elephants, pangolins, and certain reptile species are acquired through legal and illegal trade operations in order to continue these historic cultural healing practices. Within the last decade nearly 975 different wild animal taxa groups have been legally and illegally exported out of Africa and imported into areas like China, Japan, Indonesia, the United States, Russia, Europe, and South America.

Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the strain of coronavirus that causes coronavirus disease 2019 (COVID-19), the respiratory illness responsible for the COVID-19 pandemic. Colloquially known as simply the coronavirus, it was previously referred to by its provisional name, 2019 novel coronavirus (2019-nCoV), and has also been called human coronavirus 2019. The World Health Organization declared the outbreak a Public Health Emergency of International Concern on 30 January 2020, and a pandemic on 11 March 2020.

Coronavirus disease 2019 (COVID-19) is a contagious respiratory and vascular disease. It is caused by becoming infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is a specific type of coronavirus. Common symptoms include fever, cough, fatigue, shortness of breath or breathing difficulties, and loss of smell and taste. The incubation period, which is the time between becoming infected with the virus and showing symptoms, may range from one to fourteen days. While most people have mild symptoms, some people develop acute respiratory distress syndrome (ARDS) possibly precipitated by cytokine storm, multi-organ failure, septic shock, and blood clots. Longer-term damage to organs has been observed, and there is concern about a significant number of patients who have recovered from the acute phase of the disease but continue to experience a range of effects—including severe fatigue, memory loss and other cognitive issues, low grade fever, muscle weakness, breathlessness, and other symptoms—for months afterwards.
Allison McGeer is a Canadian infectious disease specialist in the Sinai Health System, a Professor at the Dalla Lana School of Public Health and a Senior Clinician Scientist at the Lunenfeld-Tanenbaum Research Institute. McGeer has led investigations into the severe acute respiratory syndrome outbreak in Toronto and worked alongside Donald Low. During the COVID-19 pandemic, McGeer has studied how SARS-CoV-2 survives in the air.
Natalie E. Dean is an American biostatistician specializing in infectious disease epidemiology. Dean is currently an assistant professor of Biostatistics at the University of Florida. Her research involves epidemiological modeling of outbreaks, including Ebola, Zika and coronavirus disease.
COVID-19 spreads from person to person, mainly through the respiratory route, after an infected person coughs, sneezes, sings, talks or breathes. A new infection occurs when droplets or virus containing particles get into the mouth, nose, lungs or eyes of other people who are in close contact with the infected person. Both droplets and aerosols cause infection when people are physically near, and the closer people interact, the more likely they are to be infected. Aerosols, however, remain suspended in the air for longer periods of time, causing airborne transmission particularly in crowded and inadequately ventilated indoor spaces, such as restaurants, choirs, gyms, nightclubs, offices and religious venues. Aerosols are also generated in healthcare settings.
















