Proline (symbol Pro or P) is an organic acid classed as a proteinogenic amino acid (used in the biosynthesis of proteins), although it does not contain the amino group -NH
2 but is rather a secondary amine. The secondary amine nitrogen is in the protonated form (NH2+) under biological conditions, while the carboxyl group is in the deprotonated −COO− form. The "side chain" from the α carbon connects to the nitrogen forming a pyrrolidine loop, classifying it as a aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it from the non-essential amino acid L-glutamate. It is encoded by all the codons starting with CC (CCU, CCC, CCA, and CCG).
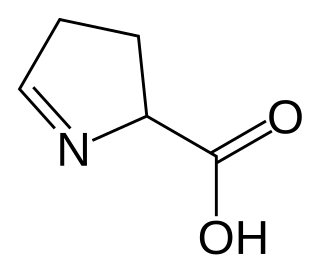
In organic chemistry, an imino acid is any molecule that contains both imine (>C=NH) and carboxyl functional groups.

1-Pyrroline-5-carboxylic acid is a cyclic imino acid. Its conjugate base and anion is 1-pyrroline-5-carboxylate (P5C). In solution, P5C is in spontaneous equilibrium with glutamate-5-semialdhyde (GSA).

Hyperprolinemia is a condition which occurs when the amino acid proline is not broken down properly by the enzymes proline oxidase or pyrroline-5-carboxylate dehydrogenase, causing a buildup of proline in the body.
In enzymology, a 1-pyrroline-5-carboxylate dehydrogenase (EC 1.2.1.88) is an enzyme that catalyzes the chemical reaction

In enzymology, proline dehydrogenase (PRODH) (EC 1.5.5.2, formerly EC 1.5.99.8) is an enzyme of the oxidoreductase family, active in the oxidation of L-proline to (S)-1-pyrroline-5-carboxylate during proline catabolism. The end product of this reaction is then further oxidized in a (S)-1-pyrroline-5-carboxylate dehydrogenase (P5CDH)-dependent reaction of the proline metabolism, or spent to produce ornithine, a crucial metabolite of ornithine and arginine metabolism. The systematic name of this enzyme class is L-proline:quinone oxidoreductase. Other names in common use include L-proline dehydrogenase, L-proline oxidase,and L-proline:(acceptor) oxidoreductase. It employs one cofactor, FAD, which requires riboflavin (vitamin B2).
In enzymology, a pyrroline-2-carboxylate reductase (EC 1.5.1.1) is an enzyme that catalyzes the chemical reaction

In enzymology, a pyrroline-5-carboxylate reductase (EC 1.5.1.2) is an enzyme that catalyzes the chemical reaction
In enzymology, a 4-hydroxyproline epimerase is an enzyme that catalyzes the chemical reaction
The enzyme 2-dehydro-3-deoxy-L-arabinonate dehydratase (EC 4.2.1.43) catalyzes the chemical reaction

The enzyme 3-dehydroquinate dehydratase (EC 4.2.1.10) catalyzes the chemical reaction
The enzyme dihydroxy-acid dehydratase (EC 4.2.1.9) catalyzes the chemical reaction
The enzyme L-arabinonate dehydratase (EC 4.2.1.25) catalyzes the chemical reaction
The enzyme L(+)-tartrate dehydratase (EC 4.2.1.32) catalyzes the chemical reaction
The enzyme (S)-2-methylmalate dehydratase (EC 4.2.1.34) catalyzes the chemical reaction:
In enzymology, a 1-pyrroline-4-hydroxy-2-carboxylate deaminase (EC 3.5.4.22) is an enzyme that catalyzes the chemical reaction

Delta-1-pyrroline-5-carboxylate dehydrogenase, mitochondrial is an enzyme that in humans is encoded by the ALDH4A1 gene.
Delta-1-pyrroline-5-carboxylate synthetase (P5CS) is an enzyme that in humans is encoded by the ALDH18A1 gene. This gene is a member of the aldehyde dehydrogenase family and encodes a bifunctional ATP- and NADPH-dependent mitochondrial enzyme with both gamma-glutamyl kinase and gamma-glutamyl phosphate reductase activities. The encoded protein catalyzes the reduction of glutamate to delta1-pyrroline-5-carboxylate, a critical step in the de novo biosynthesis of proline, ornithine and arginine. Mutations in this gene lead to hyperammonemia, hypoornithinemia, hypocitrullinemia, hypoargininemia and hypoprolinemia and may be associated with neurodegeneration, cataracts and connective tissue diseases. Alternatively spliced transcript variants, encoding different isoforms, have been described for this gene. As reported by Bruno Reversade and colleagues, ALDH18A1 deficiency or dominant-negative mutations in P5CS in humans causes a progeroid disease known as De Barsy Syndrome.
Arginine and proline metabolism is one of the central pathways for the biosynthesis of the amino acids arginine and proline from glutamate. The pathways linking arginine, glutamate, and proline are bidirectional. Thus, the net utilization or production of these amino acids is highly dependent on cell type and developmental stage. Altered proline metabolism has been linked to metastasis formation in breast cancer.







