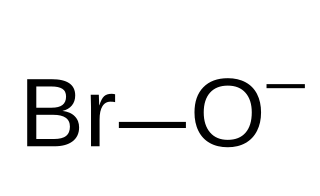Bromine is a chemical element; it has symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig and Antoine Jérôme Balard, its name was derived from the Ancient Greek βρῶμος (bromos) meaning "stench", referring to its sharp and pungent smell.

Vanadium is a chemical element; it has symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an oxide layer (passivation) somewhat stabilizes the free metal against further oxidation.
The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. This kind of conversion is in fact so common that a comprehensive overview is challenging. This article mainly deals with halogenation using elemental halogens. Halides are also commonly introduced using salts of the halides and halogen acids. Many specialized reagents exist for and introducing halogens into diverse substrates, e.g. thionyl chloride.
In organic chemistry, an aryl halide is an aromatic compound in which one or more hydrogen atoms, directly bonded to an aromatic ring are replaced by a halide. The haloarene are different from haloalkanes because they exhibit many differences in methods of preparation and properties. The most important members are the aryl chlorides, but the class of compounds is so broad that there are many derivatives and applications.

Hydrogen bromide is the inorganic compound with the formula HBr. It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at 124.3 °C. Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached.
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardant materials, and cell stains. Although uncommon, chronic toxicity from bromide can result in bromism, a syndrome with multiple neurological symptoms. Bromide toxicity can also cause a type of skin eruption, see potassium bromide. The bromide ion has an ionic radius of 196 pm.

1-Bromo-3-chloro-5,5-dimethylhydantoin is a chemical structurally related to hydantoin. It is a white crystalline compound with a slight bromine and acetone odor and is insoluble in water, but soluble in acetone.

Hypobromous acid is a weak, unstable acid with chemical formula of HOBr. It is mainly produced and handled in an aqueous solution. It is generated both biologically and commercially as a disinfectant. Salts of hypobromite are rarely isolated as solids.
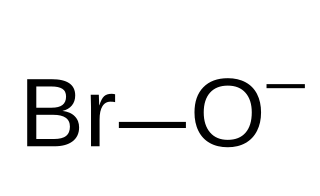
The hypobromite ion, also called alkaline bromine water, is BrO−. Bromine is in the +1 oxidation state. The Br–O bond length is 1.82 Å. Hypobromite is the bromine compound analogous to hypochlorites found in common bleaches, and in immune cells. In many ways, hypobromite functions in the same manner as hypochlorite, and is also used as a germicide and antiparasitic in both industrial applications, and in the immune system.

Vanadium compounds are compounds formed by the element vanadium (V). The chemistry of vanadium is noteworthy for the accessibility of the four adjacent oxidation states 2–5, whereas the chemistry of the other group 5 elements, niobium and tantalum, are somewhat more limited to the +5 oxidation state. In aqueous solution, vanadium forms metal aquo complexes of which the colours are lilac [V(H2O)6]2+, green [V(H2O)6]3+, blue [VO(H2O)5]2+, yellow-orange oxides [VO(H2O)5]3+, the formula for which depends on pH. Vanadium(II) compounds are reducing agents, and vanadium(V) compounds are oxidizing agents. Vanadium(IV) compounds often exist as vanadyl derivatives, which contain the VO2+ center.
Bromine compounds are compounds containing the element bromine (Br). These compounds usually form the -1, +1, +3 and +5 oxidation states. Bromine is intermediate in reactivity between chlorine and iodine, and is one of the most reactive elements. Bond energies to bromine tend to be lower than those to chlorine but higher than those to iodine, and bromine is a weaker oxidising agent than chlorine but a stronger one than iodine. This can be seen from the standard electrode potentials of the X2/X− couples (F, +2.866 V; Cl, +1.395 V; Br, +1.087 V; I, +0.615 V; At, approximately +0.3 V). Bromination often leads to higher oxidation states than iodination but lower or equal oxidation states to chlorination. Bromine tends to react with compounds including M–M, M–H, or M–C bonds to form M–Br bonds.
Chloride peroxidase (EC 1.11.1.10) is a family of enzymes that catalyzes the chlorination of organic compounds. This enzyme combines the inorganic substrates chloride and hydrogen peroxide to produce the equivalent of Cl+, which replaces a proton in hydrocarbon substrate:

Iodine oxides are chemical compounds of oxygen and iodine. Iodine has only two stable oxides which are isolatable in bulk, iodine tetroxide and iodine pentoxide, but a number of other oxides are formed in trace quantities or have been hypothesized to exist. The chemistry of these compounds is complicated with only a few having been well characterized. Many have been detected in the atmosphere and are believed to be particularly important in the marine boundary layer.

Bromous acid is the inorganic compound with the formula of HBrO2. It is an unstable compound, although salts of its conjugate base – bromites – have been isolated. In acidic solution, bromites decompose to bromine.
Haloperoxidases are peroxidases that are able to mediate the oxidation of halides by hydrogen peroxide. Both halides and hydrogen peroxide are widely available in the environment.
Organobromine chemistry is the study of the synthesis and properties of organobromine compounds, also called organobromides, which are organic compounds that contain carbon bonded to bromine. The most pervasive is the naturally produced bromomethane.

Bromide peroxidase (EC 1.11.1.18, bromoperoxidase, haloperoxidase (ambiguous), eosinophil peroxidase) is a family of enzymes with systematic name bromide:hydrogen-peroxide oxidoreductase. These enzymes catalyse the following chemical reaction:

Vincent L. Pecoraro, professor at the University of Michigan, is a researcher in bioinorganic chemistry and inorganic chemistry. He is a specialist in the chemistry and biochemistry of manganese, vanadium, and metallacrown chemistry. He is a fellow of the American Association for the Advancement of Science
A large fraction of the chemical elements that occur naturally on the Earth's surface are essential to the structure and metabolism of living things. Four of these elements are essential to every living thing and collectively make up 99% of the mass of protoplasm. Phosphorus and sulfur are also common essential elements, essential to the structure of nucleic acids and amino acids, respectively. Chlorine, potassium, magnesium, calcium and phosphorus have important roles due to their ready ionization and utility in regulating membrane activity and osmotic potential. The remaining elements found in living things are primarily metals that play a role in determining protein structure. Examples include iron, essential to hemoglobin; and magnesium, essential to chlorophyll. Some elements are essential only to certain taxonomic groups of organisms, particularly the prokaryotes. For instance, the lanthanide series rare earths are essential for methanogens. As shown in the following table, there is strong evidence that 19 of the elements are essential to all living things, and another 17 are essential to some taxonomic groups. Of these 17, most have not been extensively studied, and their biological importance may be greater than currently supposed.