
An adiabatic process is a type of thermodynamic process that occurs without transferring heat or mass between the thermodynamic system and its environment. Unlike an isothermal process, an adiabatic process transfers energy to the surroundings only as work. As a key concept in thermodynamics, the adiabatic process supports the theory that explains the first law of thermodynamics. The opposite term to "adiabatic" is diabatic.

The dew point of a given body of air is the temperature to which it must be cooled to become saturated with water vapor. This temperature depends on the pressure and water content of the air. When the air is cooled below the dew point, its moisture capacity is reduced and airborne water vapor will condense to form liquid water known as dew. When this occurs through the air's contact with a colder surface, dew will form on that surface.

The heat index (HI) is an index that combines air temperature and relative humidity, in shaded areas, to posit a human-perceived equivalent temperature, as how hot it would feel if the humidity were some other value in the shade. For example, when the temperature is 32 °C (90 °F) with 70% relative humidity, the heat index is 41 °C (106 °F). The heat index is meant to describe experienced temperatures in the shade, but it does not take into account heating from direct sunlight, physical activity or cooling from wind.

The first law of thermodynamics is a formulation of the law of conservation of energy in the context of thermodynamic processes. The law distinguishes two principal forms of energy transfer, heat and thermodynamic work, that modify a thermodynamic system containing a constant amount of matter. The law also defines the internal energy of a system, an extensive property for taking account of the balance of heat and work in the system. Energy cannot be created or destroyed, but it can be transformed from one form to another. In an isolated system the sum of all forms of energy is constant.

A hygrometer is an instrument which measures the humidity of air or some other gas: that is, how much of it is water vapor. Humidity measurement instruments usually rely on measurements of some other quantities such as temperature, pressure, mass, and mechanical or electrical changes in a substance as moisture is absorbed. By calibration and calculation, these measured quantities can be used to indicate the humidity. Modern electronic devices use the temperature of condensation, or they sense changes in electrical capacitance or resistance.
Equivalent potential temperature, commonly referred to as theta-e, is a quantity that is conserved during changes to an air parcel's pressure, even if water vapor condenses during that pressure change. It is therefore more conserved than the ordinary potential temperature, which remains constant only for unsaturated vertical motions.

Alpine climate is the typical climate for elevations above the tree line, where trees fail to grow due to cold. This climate is also referred to as a mountain climate or highland climate.

An evaporative cooler is a device that cools air through the evaporation of water. Evaporative cooling differs from other air conditioning systems, which use vapor-compression or absorption refrigeration cycles. Evaporative cooling exploits the fact that water will absorb a relatively large amount of heat in order to evaporate. The temperature of dry air can be dropped significantly through the phase transition of liquid water to water vapor (evaporation). This can cool air using much less energy than refrigeration. In extremely dry climates, evaporative cooling of air has the added benefit of conditioning the air with more moisture for the comfort of building occupants.

A cooling tower is a device that rejects waste heat to the atmosphere through the cooling of a coolant stream, usually a water stream, to a lower temperature. Cooling towers may either use the evaporation of water to remove heat and cool the working fluid to near the wet-bulb air temperature or, in the case of dry cooling towers, rely solely on air to cool the working fluid to near the dry-bulb air temperature using radiators.

Psychrometrics is the field of engineering concerned with the physical and thermodynamic properties of gas-vapor mixtures.

The wet-bulb globe temperature (WBGT) is a measure of environmental heat as it affects humans. Unlike a simple temperature measurement, WBGT accounts for all four major environmental heat factors: air temperature, humidity, radiant heat, and air movement. It is used by industrial hygienists, athletes, sporting events and the military to determine appropriate exposure levels to high temperatures.
The potential temperature of a parcel of fluid at pressure is the temperature that the parcel would attain if adiabatically brought to a standard reference pressure , usually 1,000 hPa (1,000 mb). The potential temperature is denoted and, for a gas well-approximated as ideal, is given by

Thermodynamic work is one of the principal kinds of process by which a thermodynamic system can interact with and transfer energy to its surroundings. This results in externally measurable macroscopic forces on the system's surroundings, which can cause mechanical work, to lift a weight, for example, or cause changes in electromagnetic, or gravitational variables. Also, the surroundings can perform thermodynamic work on a thermodynamic system, which is measured by an opposite sign convention.

The wet-bulb temperature (WBT) is a temperature that can be measured by a thermometer covered in cloth which has been soaked in water at ambient temperature and over which air is passed. At 100% relative humidity, the wet-bulb temperature is equal to the air temperature ; at lower humidity the wet-bulb temperature is lower than dry-bulb temperature because of evaporative cooling.
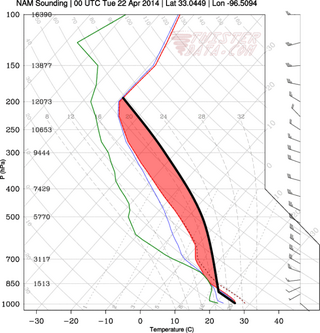
In meteorology, convective instability or stability of an air mass refers to its ability to resist vertical motion. A stable atmosphere makes vertical movement difficult, and small vertical disturbances dampen out and disappear. In an unstable atmosphere, vertical air movements tend to become larger, resulting in turbulent airflow and convective activity. Instability can lead to significant turbulence, extensive vertical clouds, and severe weather such as thunderstorms.
In atmospheric science, equivalent temperature is the temperature of air in a parcel from which all the water vapor has been extracted by an adiabatic process.
Atmospheric thermodynamics is the study of heat-to-work transformations that take place in the Earth's atmosphere and manifest as weather or climate. Atmospheric thermodynamics use the laws of classical thermodynamics, to describe and explain such phenomena as the properties of moist air, the formation of clouds, atmospheric convection, boundary layer meteorology, and vertical instabilities in the atmosphere. Atmospheric thermodynamic diagrams are used as tools in the forecasting of storm development. Atmospheric thermodynamics forms a basis for cloud microphysics and convection parameterizations used in numerical weather models and is used in many climate considerations, including convective-equilibrium climate models.

Apparent temperature, also known as "feels like", is the temperature equivalent perceived by humans, caused by the combined effects of air temperature, relative humidity and wind speed. The measure is most commonly applied to the perceived outdoor temperature. Apparent temperature was invented by Robert G. Steadman who published a paper about it in 1984. It also applies, however, to indoor temperatures, especially saunas, and when houses and workplaces are not sufficiently heated or cooled.

The rear flank downdraft (RFD) is a region of dry air wrapping around the back of a mesocyclone in a supercell thunderstorm. These areas of descending air are thought to be essential in the production of many supercellular tornadoes. Large hail within the rear flank downdraft often shows up brightly as a hook on weather radar images, producing the characteristic hook echo, which often indicates the presence of a tornado.

In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by modes other than thermodynamic work and transfer of matter. Such modes are microscopic, mainly thermal conduction, radiation, and friction, as distinct from the macroscopic modes, thermodynamic work and transfer of matter. For a closed system, the heat involved in a process is the difference in internal energy between the final and initial states of a system, and subtracting the work done in the process. For a closed system, this is the formulation of the first law of thermodynamics.












