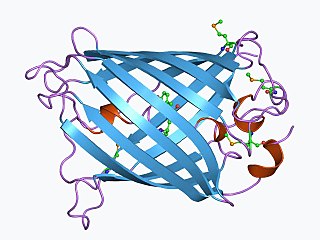
The green fluorescent protein (GFP) is a protein that exhibits green fluorescence when exposed to light in the blue to ultraviolet range. The label GFP traditionally refers to the protein first isolated from the jellyfish Aequorea victoria and is sometimes called avGFP. However, GFPs have been found in other organisms including corals, sea anemones, zoanithids, copepods and lancelets.
Phycobilins are light-capturing bilins found in cyanobacteria and in the chloroplasts of red algae, glaucophytes and some cryptomonads. Most of their molecules consist of a chromophore which makes them coloured. They are unique among the photosynthetic pigments in that they are bonded to certain water-soluble proteins, known as phycobiliproteins. Phycobiliproteins then pass the light energy to chlorophylls for photosynthesis.

Phytochromes are a class of photoreceptor proteins found in plants, bacteria and fungi. They respond to light in the red and far-red regions of the visible spectrum and can be classed as either Type I, which are activated by far-red light, or Type II that are activated by red light. Recent advances have suggested that phytochromes also act as temperature sensors, as warmer temperatures enhance their de-activation. All of these factors contribute to the plant's ability to germinate.
Phycoerythrin (PE) is a red protein-pigment complex from the light-harvesting phycobiliprotein family, present in cyanobacteria, red algae and cryptophytes, accessory to the main chlorophyll pigments responsible for photosynthesis.The red pigment is due to the prosthetic group, phycoerythrobilin, which gives phycoerythrin its red color.
Photopigments are unstable pigments that undergo a chemical change when they absorb light. The term is generally applied to the non-protein chromophore moiety of photosensitive chromoproteins, such as the pigments involved in photosynthesis and photoreception. In medical terminology, "photopigment" commonly refers to the photoreceptor proteins of the retina.
In developmental biology, photomorphogenesis is light-mediated development, where plant growth patterns respond to the light spectrum. This is a completely separate process from photosynthesis where light is used as a source of energy. Phytochromes, cryptochromes, and phototropins are photochromic sensory receptors that restrict the photomorphogenic effect of light to the UV-A, UV-B, blue, and red portions of the electromagnetic spectrum.
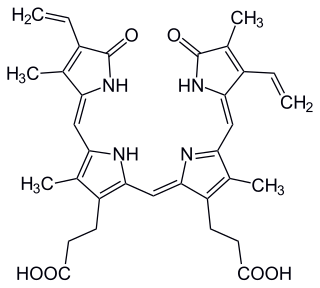
Biliverdin is a green tetrapyrrolic bile pigment, and is a product of heme catabolism. It is the pigment responsible for a greenish color sometimes seen in bruises.
Accessory pigments are light-absorbing compounds, found in photosynthetic organisms, that work in conjunction with chlorophyll a. They include other forms of this pigment, such as chlorophyll b in green algal and vascular ("higher") plant antennae, while other algae may contain chlorophyll c or d. In addition, there are many non-chlorophyll accessory pigments, such as carotenoids or phycobiliproteins, which also absorb light and transfer that light energy to photosystem chlorophyll. Some of these accessory pigments, in particular the carotenoids, also serve to absorb and dissipate excess light energy, or work as antioxidants. The large, physically associated group of chlorophylls and other accessory pigments is sometimes referred to as a pigment bed.

Phycocyanin is a pigment-protein complex from the light-harvesting phycobiliprotein family, along with allophycocyanin and phycoerythrin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble, so they cannot exist within the membrane like carotenoids can. Instead, phycobiliproteins aggregate to form clusters that adhere to the membrane called phycobilisomes. Phycocyanin is a characteristic light blue color, absorbing orange and red light, particularly 620 nm, and emits fluorescence at about 650 nm. Allophycocyanin absorbs and emits at longer wavelengths than phycocyanin C or phycocyanin R. Phycocyanins are found in cyanobacteria. Phycobiliproteins have fluorescent properties that are used in immunoassay kits. Phycocyanin is from the Greek phyco meaning “algae” and cyanin is from the English word “cyan", which conventionally means a shade of blue-green and is derived from the Greek “kyanos" which means a somewhat different color: "dark blue". The product phycocyanin, produced by Aphanizomenon flos-aquae and Spirulina, is for example used in the food and beverage industry as the natural coloring agent 'Lina Blue' or 'EXBERRY Shade Blue' and is found in sweets and ice cream. In addition, fluorescence detection of phycocyanin pigments in water samples is a useful method to monitor cyanobacteria biomass.
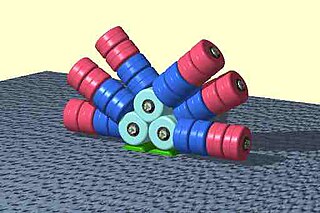
Phycobilisomes are light-harvesting antennae that transmit the energy of harvested photons to photosystem II and photosystem I in cyanobacteria and in the chloroplasts of red algae and glaucophytes. They were lost during the evolution of the chloroplasts of green algae and plants.

Allophycocyanin is a protein from the light-harvesting phycobiliprotein family, along with phycocyanin, phycoerythrin and phycoerythrocyanin. It is an accessory pigment to chlorophyll. All phycobiliproteins are water-soluble and therefore cannot exist within the membrane like carotenoids, but aggregate, forming clusters that adhere to the membrane called phycobilisomes. Allophycocyanin absorbs and emits red light, and is readily found in Cyanobacteria, and red algae. Phycobilin pigments have fluorescent properties that are used in immunoassay kits. In flow cytometry, it is often abbreviated APC. To be effectively used in applications such as FACS, High-Throughput Screening (HTS) and microscopy, APC needs to be chemically cross-linked.

Phycobiliproteins are water-soluble proteins present in cyanobacteria and certain algae. They capture light energy, which is then passed on to chlorophylls during photosynthesis. Phycobiliproteins are formed of a complex between proteins and covalently bound phycobilins that act as chromophores. They are most important constituents of the phycobilisomes.
A light-harvesting complex consists of a number of chromophores which are complex subunit proteins that may be part of a larger super complex of a photosystem, the functional unit in photosynthesis. It is used by plants and photosynthetic bacteria to collect more of the incoming light than would be captured by the photosynthetic reaction center alone. The light which is captured by the chromophores is capable of exciting molecules from their ground state to a higher energy state, known as the excited state. This excited state does not last very long and is known to be short-lived.

Bilins, bilanes or bile pigments are biological pigments formed in many organisms as a metabolic product of certain porphyrins. Bilin was named as a bile pigment of mammals, but can also be found in lower vertebrates, invertebrates, as well as red algae, green plants and cyanobacteria. Bilins can range in color from red, orange, yellow or brown to blue or green.
Photoreceptor proteins are light-sensitive proteins involved in the sensing and response to light in a variety of organisms. Some examples are rhodopsin in the photoreceptor cells of the vertebrate retina, phytochrome in plants, and bacteriorhodopsin and bacteriophytochromes in some bacteria. They mediate light responses as varied as visual perception, phototropism and phototaxis, as well as responses to light-dark cycles such as circadian rhythm and other photoperiodisms including control of flowering times in plants and mating seasons in animals.
Phycoerythrocyanin is a kind of phycobiliprotein, magenta chromoprotein involved in photosynthesis of some Cyanobacteria. This chromoprotein consists of alpha- and beta-subunits, generally aggregated as hexamer. Alpha-phycoerythrocyanin contains a phycoviolobilin, a violet bilin, that covalently attached at Cys-84, and beta-phycoerythrocyanin contains two phycocyanobilins, a blue bilin, that covalently attached at Cys-84 and -155, respectively. Phycoerythrocyanin is similar to phycocyanin, an important component of the light-harvesting complex (phycobilisome) of cyanobacteria and red algae.
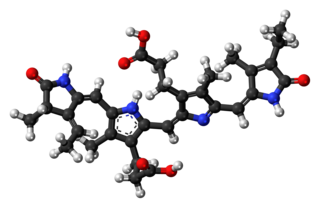
Phycocyanobilin is a blue phycobilin, i.e., a tetrapyrrole chromophore found in cyanobacteria and in the chloroplasts of red algae, glaucophytes, and some cryptomonads. Phycocyanobilin is present only in the phycobiliproteins allophycocyanin and phycocyanin, of which it is the terminal acceptor of energy. It is covalently linked to these phycobiliproteins by a thioether bond.
In enzymology, a phycocyanobilin:ferredoxin oxidoreductase is an enzyme that catalyzes the chemical reaction

Small ultra red fluorescent protein (smURFP) is a class of far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein, α-allophycocyanin. Native α-allophycocyanin requires an exogenous protein, known as a lyase, to attach the chromophore, phycocyanobilin. Phycocyanobilin is not present in mammalian cells. smURFP was evolved to covalently attach phycocyanobilin without a lyase and fluoresce, covalently attach biliverdin and fluoresce, blue-shift fluorescence to match the organic fluorophore, Cy5, and not inhibit E. coli growth. smURFP was found after 12 rounds of random mutagenesis and manually screening 10,000,000 bacterial colonies.
Alexander Glazer was a professor of the Graduate School in the Department of Molecular and Cell Biology at the University of California, Berkeley. He had a passion for protein chemistry and structure function relationships. He also had a longstanding interest in light-harvesting complexes in cyanobacteria and red algae called phycobilisomes. He had also spent more than 10 years working on the human genome project where he has investigated methods for DNA detection and sequencing which most notably includes the development of fluorescent reagents involved in cell labeling. Most recently, he had focused his studies on issues in environmental sciences. He died on July 18, 2021, in Orinda, California












