
Triphenylphosphine (IUPAC name: triphenylphosphane) is a common organophosphorus compound with the formula P(C6H5)3 and often abbreviated to PPh3 or Ph3P. It is versatile compound that is widely used as a reagent in organic synthesis and as a ligand for transition metal complexes, including ones that serve as catalysts in organometallic chemistry. PPh3 exists as relatively air stable, colorless crystals at room temperature. It dissolves in non-polar organic solvents such as benzene and diethyl ether.

Rhodium(III) chloride refers to inorganic compounds with the formula RhCl3(H2O)n, where n varies from 0 to 3. These are diamagnetic red-brown solids. The soluble trihydrated (n = 3) salt is the usual compound of commerce. It is widely used to prepare compounds used in homogeneous catalysis.

Palladium(II) chloride, also known as palladium dichloride and palladous chloride, are the chemical compounds with the formula PdCl2. PdCl2 is a common starting material in palladium chemistry – palladium-based catalysts are of particular value in organic synthesis. It is prepared by the reaction of chlorine with palladium metal at high temperatures.
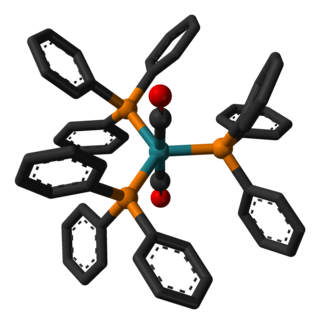
Dicarbonyltris(triphenylphosphine)ruthenium(0) or Roper's complex is a ruthenium metal carbonyl. In it, two carbon monoxide ligands and three triphenylphosphine ligands are coordinated to a central ruthenium(0) center.
1,5-Cyclooctadiene is a cyclic hydrocarbon with the chemical formula C8H12, specifically [−(CH2)2−CH=CH−]2.

Zeise's salt, potassium trichloro(ethylene)platinate(II) hydrate, is the chemical compound with the formula K[PtCl3(C2H4)]·H2O. The anion of this air-stable, yellow, coordination complex contains an η2-ethylene ligand. The anion features a platinum atom with a square planar geometry. The salt is of historical importance in the area of organometallic chemistry as one of the first examples of a transition metal alkene complex and is named for its discoverer, William Christopher Zeise.

Chloro(triphenylphosphine)gold(I) or triphenylphosphinegold(I) chloride is a coordination complex with the formula (Ph3P)AuCl. This colorless solid is a common reagent for research on gold compounds.
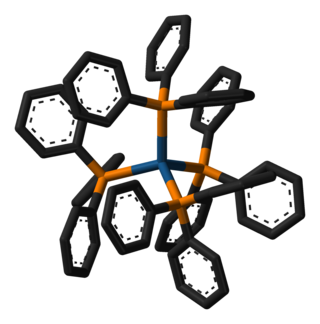
Tetrakis(triphenylphosphine)platinum(0) is the chemical compound with the formula Pt(P(C6H5)3)4, often abbreviated Pt(PPh3)4. The bright yellow compound is used as a precursor to other platinum complexes.

Potassium tetrachloroplatinate(II) is the chemical compound with the formula K2PtCl4. This reddish orange salt is an important reagent for the preparation of other coordination complexes of platinum. It consists of potassium cations and the square planar dianion PtCl42−. Related salts are also known including Na2PtCl4, which is brown-colored and soluble in alcohols, and quaternary ammonium salts, which are soluble in a broader range of organic solvents.
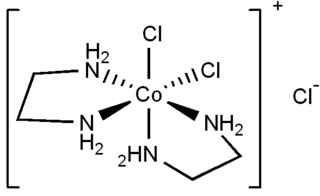
cis-Dichlorobis(ethylenediamine)cobalt(III) chloride is a salt with the formula [CoCl2(en)2]Cl (en = ethylenediamine). The salt consists of a cationic coordination complex and a chloride anion. It is a violet diamagnetic solid that is soluble in water. One chloride ion in this salt readily undergoes ion exchange, but the two other chlorides are less reactive, being bound to the metal center.

Bis(triphenylphosphine)palladium chloride is a coordination compound of palladium containing two triphenylphosphine and two chloride ligands. It is a yellow solid that is soluble in some organic solvents. It is used for palladium-catalyzed coupling reactions, e.g. the Sonogashira–Hagihara reaction. The complex is square planar. Many analogous complexes are known with different phosphine ligands.
Organoplatinum chemistry is the chemistry of organometallic compounds containing a carbon to platinum chemical bond, and the study of platinum as a catalyst in organic reactions. Organoplatinum compounds exist in oxidation state 0 to IV, with oxidation state II most abundant. The general order in bond strength is Pt-C (sp) > Pt-O > Pt-N > Pt-C (sp3). Organoplatinum and organopalladium chemistry are similar, but organoplatinum compounds are more stable and therefore less useful as catalysts.

Dichlorotris(triphenylphosphine)ruthenium(II) is a coordination complex of ruthenium. It is a chocolate brown solid that is soluble in organic solvents such as benzene. The compound is used as a precursor to other complexes including those used in homogeneous catalysis.

A metal-phosphine complex is a coordination complex containing one or more phosphine ligands. Almost always, the phosphine is an organophosphine of the type R3P (R = alkyl, aryl). Metal phosphine complexes are useful in homogeneous catalysis. Prominent examples of metal phosphine complexes include Wilkinson's catalyst (Rh(PPh3)3Cl), Grubbs' catalyst, and tetrakis(triphenylphosphine)palladium(0).

Carbonyl hydrido tris(triphenylphosphine)rhodium(I) [Carbonyl(hydrido)tris(triphenylphosphane)rhodium(I)] is an organorhodium compound with the formula [RhH(CO)(PPh3)3] (Ph = C6H5). It is a yellow, benzene-soluble solid, which is used industrially for hydroformylation.

Dichlorobis(triphenylphosphine)nickel(II) refers to a pair of metal phosphine complexes with the formula NiCl2[P(C6H5)3]2. The compound exists as two isomers, a paramagnetic dark blue solid and a diamagnetic red solid. These complexes function as catalysts for organic synthesis.
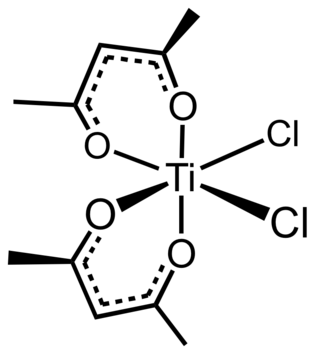
Titanium bis(acetylacetonate)dichloride is the coordination complex with the formula Ti(C5H7O2)2Cl2. It is a common acetylacetonate complex of titanium. It is a red-orange solid that hydrolyzes slowly in air.

cis-Dichlorobis(bipyridine)ruthenium(II) is the coordination complex with the formula RuCl2(bipy)2, where bipy is 2,2'-bipyridine. It is a dark green diamagnetic solid that is a precursor to many other complexes of ruthenium, mainly by substitution of the two chloride ligands. The compound has been crystallized as diverse hydrates.

Transition metal thioether complexes comprise coordination complexes of thioether (R2S) ligands. The inventory is extensive.
Transition metal complexes of phosphine oxides are coordination complex containing one or more phosphine oxide ligands. Many phosphine oxides exist and most behave as hard Lewis bases. Almost invariably, phosphine oxides bind metals by formation of M-O bonds.





















