Related Research Articles

Inorganic chemistry deals with synthesis and behavior of inorganic and organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subjects of organic chemistry. The distinction between the two disciplines is far from absolute, as there is much overlap in the subdiscipline of organometallic chemistry. It has applications in every aspect of the chemical industry, including catalysis, materials science, pigments, surfactants, coatings, medications, fuels, and agriculture.
In chemistry, the oxidation state, or oxidation number, is the hypothetical charge of an atom if all of its bonds to other atoms were fully ionic. It describes the degree of oxidation of an atom in a chemical compound. Conceptually, the oxidation state may be positive, negative or zero. While fully ionic bonds are not found in nature, many bonds exhibit strong ionicity, making oxidation state a useful predictor of charge.

Jack Lewis, Baron Lewis of Newnham, FRS, HonFRSC was an English chemist working mainly in the area of inorganic chemistry.

Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored liquid with a pungent odour. Older samples appear darker. This compound is a common precursor to diverse iron compounds, including many that are useful in small scale organic synthesis.

Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts or catalyst precursors in homogeneous catalysis, such as hydroformylation and Reppe chemistry. In the Mond process, nickel tetracarbonyl is used to produce pure nickel. In organometallic chemistry, metal carbonyls serve as precursors for the preparation of other organometallic complexes.
Osmium compounds are compounds containing the element osmium (Os). Osmium forms compounds with oxidation states ranging from −2 to +8. The most common oxidation states are +2, +3, +4, and +8. The +8 oxidation state is notable for being the highest attained by any chemical element aside from iridium's +9 and is encountered only in xenon, ruthenium, hassium, iridium, and plutonium. The oxidation states −1 and −2 represented by the two reactive compounds Na
2[Os
4(CO)
13] and Na
2[Os(CO)
4] are used in the synthesis of osmium cluster compounds.
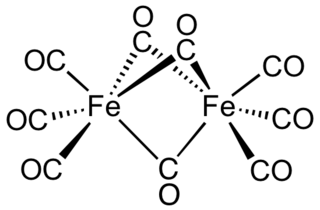
Diiron nonacarbonyl is an organometallic compound with the formula Fe2(CO)9. This metal carbonyl is an important reagent in organometallic chemistry and of occasional use in organic synthesis. It is a more reactive source of Fe(0) than Fe(CO)5. This micaceous orange solid is virtually insoluble in all common solvents.

Dimanganese decacarbonyl, which has the chemical formula Mn2(CO)10, is a binary bimetallic carbonyl complex centered around the first row transition metal manganese. The first reported synthesis of Mn2(CO)10 was in 1954 at Linde Air Products Company and was performed by Brimm, Lynch, and Sesny. Their hypothesis about, and synthesis of, dimanganese decacarbonyl was fundamentally guided by the previously known dirhenium decacarbonyl (Re2(CO)10), the heavy atom analogue of Mn2(CO)10. Since its first synthesis, Mn2(CO)10 has been use sparingly as a reagent in the synthesis of other chemical species, but has found the most use as a simple system on which to study fundamental chemical and physical phenomena, most notably, the metal-metal bond. Dimanganese decacarbonyl is also used as a classic example to reinforce fundamental topics in organometallic chemistry like d-electron count, the 18-electron rule, oxidation state, valency, and the isolobal analogy.

Metal nitrosyl complexes are complexes that contain nitric oxide, NO, bonded to a transition metal. Many kinds of nitrosyl complexes are known, which vary both in structure and coligand.

Triruthenium dodecacarbonyl is the chemical compound with the formula Ru3(CO)12. Classified as metal carbonyl cluster, it is a dark orange-colored solid that is soluble in nonpolar organic solvents. The compound serves as a precursor to other organoruthenium compounds.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.
Metal nitrido complexes are coordination compounds and metal clusters that contain an atom of nitrogen bound only to transition metals. These compounds are molecular, i.e. discrete in contrast to the polymeric, dense nitride materials that are useful in materials science. The distinction between the molecular and solid-state polymers is not always very clear as illustrated by the materials Li6MoN4 and more condensed derivatives such as Na3MoN3. Transition metal nitrido complexes have attracted interest in part because it is assumed that nitrogen fixation proceeds via nitrido intermediates. Nitrido complexes have long been known, the first example being salts of [OsO3N]−, described in the 19th century.
Transition metal carbyne complexes are organometallic compounds with a triple bond between carbon and the transition metal. This triple bond consists of a σ-bond and two π-bonds. The HOMO of the carbyne ligand interacts with the LUMO of the metal to create the σ-bond. The two π-bonds are formed when the two HOMO orbitals of the metal back-donate to the LUMO of the carbyne. They are also called metal alkylidynes—the carbon is a carbyne ligand. Such compounds are useful in organic synthesis of alkynes and nitriles. They have been the focus on much fundamental research.
A metal carbido complex is a coordination complex that contains a carbon atom as a ligand. They are analogous to metal nitrido complexes. Carbido complexes are a molecular subclass of carbides, which are prevalent in organometallic and inorganic chemistry. Carbido complexes represent models for intermediates in Fischer–Tropsch synthesis, olefin metathesis, and related catalytic industrial processes. Ruthenium-based carbido complexes are by far the most synthesized and characterized to date. Although, complexes containing chromium, gold, iron, nickel, molybdenum, osmium, rhenium, and tungsten cores are also known. Mixed-metal carbides are also known.
In Lewis acid catalysis of organic reactions, a metal-based Lewis acid acts as an electron pair acceptor to increase the reactivity of a substrate. Common Lewis acid catalysts are based on main group metals such as aluminum, boron, silicon, and tin, as well as many early and late d-block metals. The metal atom forms an adduct with a lone-pair bearing electronegative atom in the substrate, such as oxygen, nitrogen, sulfur, and halogens. The complexation has partial charge-transfer character and makes the lone-pair donor effectively more electronegative, activating the substrate toward nucleophilic attack, heterolytic bond cleavage, or cycloaddition with 1,3-dienes and 1,3-dipoles.

In chemistry, a metal carbonyl cluster is a compound that contains two or more metals linked in part by metal-metal bonds and containing carbon monoxide (CO) as the exclusive or predominant ligand. The area is a subfield of metal carbonyl chemistry, and many metal carbonyl clusters are in fact prepared from simple metal carbonyls. Simple examples include Fe2(CO)9, Fe3(CO)12, Mn2(CO)10. High nuclearity clusters include [Rh13(CO)24H3]2− and the stacked Pt3 triangules [Pt3n(CO)6n]2− (n = 2–6).
Metal arene complexes are organometallic compounds of the formula (C6R6)xMLy. Common classes are of the type (C6R6)ML3 and (C6R6)2M. These compounds are reagents in inorganic and organic synthesis. The principles that describe arene complexes extend to related organic ligands such as many heterocycles (e.g. thiophene) and polycyclic aromatic compounds (e.g. naphthalene).

Transition metal isocyanide complexes are coordination compounds containing isocyanide ligands. Because isocyanides are relatively basic, but also good pi-acceptors, a wide range of complexes are known. Some isocyanide complexes are used in medical imaging.
Gallium monoiodide (GaI or Ga4I4) is a low-valent gallium species that acts as a reactive intermediate for many gallium-based products. Gallium(I) halides were first crystallographically characterized by Schnöckel and coworkers and have allowed a synthetic route to many low-valent gallium species. However, chemical syntheses that employ “GaI” rather than gallium(I) halide precursors have been increasingly investigated given the ease of synthesis of this reagent. While the synthetic method of Schnöckel and coworkers to synthesize gallium(I) halides require extraordinarily high temperatures, the straightforward preparation of “GaI” at near room temperature has allowed for the exploration of new gallium-based chemistries.
Phosphanides are chemicals containing the [PH2]− anion. This is also known as the phosphino anion or phosphido ligand. The IUPAC name can also be dihydridophosphate(1−).
References
- 1 2 3 "JOHNSON, Prof. Brian Frederick Gilbert" . Who's Who . Vol. 2014 (online Oxford University Press ed.). A & C Black.(Subscription or UK public library membership required.)
- ↑ Lord Lewis Of Newnham; Johnson, B. F. G. (1997). "Cyril Clifford Addison. 28 November 1913--1 April 1994.: Elected F.R.S. 1970". Biographical Memoirs of Fellows of the Royal Society . 43: 3. doi: 10.1098/rsbm.1997.0001 .
- 1 2 Harvey, P. D. (2005). "Honouring Brian F. G. Johnson FRS, FRSE, FRSC, F. Acad. Europa". Journal of Cluster Science. 17: 1–3. doi:10.1007/s10876-005-0036-7. S2CID 98476514.
- ↑ The Daily Telegraph - Birthdays 11 September 2010
- ↑ Midgley, P. A.; Weyland, M.; Thomas, J. M.; Johnson, B. F. G. (2001). "Z-Contrast tomography: A technique in three-dimensional nanostructural analysis based on Rutherford scattering". Chemical Communications (10): 907–908. doi:10.1039/B101819C.
- ↑ Jackson, Peter F.; Johnson, Brian F. G.; Lewis, Jack; Nelson, William J. H.; McPartlin, Mary (1982). "The synthesis of the cluster dianion [Os10C(CO)24]2− by pyrolysis. X-Ray structure analysis of [N(PPh3)2]2[Os10C(CO)24] and [Os5C(CO)14H(NC5H4)]". Journal of the Chemical Society, Dalton Transactions (10): 2099. doi:10.1039/DT9820002099.
- ↑ "EC/1991/16: Johnson, Brian Frederick Gilbert". London: The Royal Society. Archived from the original on 5 March 2017.
